Chapter 162
Acute Ischemia
Treatment
Christopher J. Kwolek, Fahad Shuja
Based on a chapter in the seventh edition by Karthikeshwar Kasirajian
Acute limb ischemia (ALI) results from a sudden decrease in limb perfusion that threatens limb viability and often requires urgent revascularization.1 Historic rates of amputation and mortality range from 10% to 25%, emphasizing the need for prompt evaluation and treatment.2,3 The initial evaluation and decision making in the management of ALI are discussed in detail in Chapter 161. This chapter focuses on percutaneous endovascular, open surgical, and hybrid treatment options for the management of acute arterial ischemia.
Initial Management
Once the diagnosis of acute ischemia has been established and its severity classified, a number of immediate interventions are critical to optimize patient outcomes.
Anticoagulation and Supportive Measures
Systemic anticoagulation with unfractionated heparin should be initiated to minimize the risk of further clot propagation and to prevent microvascular thrombosis of underperfused distal vessels. An initial weight-based bolus of 100 mg/kg is appropriate for most patients followed by an intravenous infusion of 1000 U/hr. If urgent operation is not undertaken, the heparin dose should be titrated to maintain an activated partial thromboplastin time between 60 and 100 seconds or 2.0 to 3.0 times normal values.
Other measures that may be beneficial in patients with ALI include intravenous hydration, supplemental oxygen, and intravenous analgesia. Patients with acute ischemia are often relatively volume depleted, and careful fluid resuscitation is necessary, with fluid administration titrated to urine output. The potential for myoglobinuria due to ischemia-reperfusion, combined with the use of contrast agents during diagnosis and treatment of ALI, increases the risk of acute renal insufficiency, which may be mitigated with appropriate fluid resuscitation.
Results of a full serum chemistry panel, including blood urea nitrogen and creatinine, complete blood count, and baseline coagulation studies, will guide further therapy. In patients with recurrent thrombosis, a full hypercoagulability workup should be considered, including evaluation for a history of previous heparin exposure and the possibility of a thrombophilia due to heparin-induced thrombocytopenia.
Treatment Selection
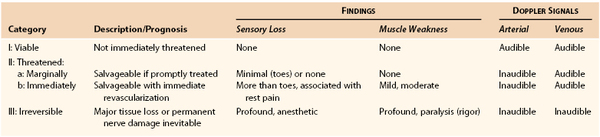
Treatment for ALI depends largely on the extent of limb ischemia present clinically. Rutherford et al4 developed a classification of ALI that is helpful in determining the appropriate therapeutic intervention and overall prognosis (Table 162-1).
Class I ALI may require only medical therapy, such as anticoagulation. Revascularization, if contemplated, can be performed electively and can consist of either thrombolytic or open surgical intervention. Treatment selection depends on the duration of ALI, the location and cause of the occlusion, the presence or absence of underlying atherosclerotic occlusive disease, and the patient’s overall medical condition.
Class II ALI requires a flexible approach to intervention. All patients with class II ALI require revascularization to preserve the functional integrity of the affected extremity. For patients with class IIa ischemia, immediate revascularization is not necessary. Consequently, either endovascular or surgical options may be considered. In the planning of therapy for a patient with class IIa ALI, the duration of symptoms is of prime importance. Percutaneous endovascular options are more effective in patients with ischemia of less than 2 weeks’ duration, whereas ischemic symptoms of more than 2 weeks’ duration are better served by surgical revascularization.5 For a duration of symptoms less than 14 days, prospective studies comparing thrombolytic and surgical intervention favor the initial use of thrombolytic therapy, with surgical intervention reserved for those limbs that do not show response to lytic therapy. Because the ischemic insult in class IIa ALI is mild, therapy may be performed on an urgent, rather than emergency, basis.
More severe ALI (class IIb), manifested by both sensory and motor deficits, requires emergency revascularization. Because time is a factor, surgical revascularization has been preferred. However, advances in catheter-based thrombolytic delivery and percutaneous mechanical thrombectomy devices have shortened the time to reperfusion. Consequently, these techniques are increasingly being used as first-line therapy in patients with class IIb ALI. In addition, the availability of hybrid operating rooms has allowed physicians to perform diagnostic imaging, endovascular intervention, and open surgical revascularization in single settings as necessary. Class III ALI manifests as a profound neurologic deficit (insensate, paretic limb), muscle rigidity, and the absence of arterial and venous Doppler ultrasound signals in the affected vascular bed. In patients with class III ALI, revascularization is usually futile, and primary amputation should be considered.
Endovascular Treatment
Endovascular procedures offer less invasive revascularization strategies for sick or elderly patients with decreased morbidity and mortality. Currently available percutaneous endovascular procedures include catheter-directed thrombolysis, pharmacomechanical thrombolysis, catheter-directed thrombus aspiration, and percutaneous mechanical thrombectomy.1,3 These techniques clear the occluding thrombus from a peripheral artery with a minimally invasive approach, restore blood flow to the extremity, and allow the identification of underlying lesions responsible for the occlusive event. Culprit lesions may then be addressed in a directed fashion with an endovascular procedure such as angioplasty, stenting, or atherectomy.
Catheter-Directed Thrombolysis
Catheter-directed thrombolysis (CDT) has become the preferred treatment in many centers for the management of most viable or marginally threatened limbs (classes I and IIA) after recent occlusive events in native arteries and for thrombosed grafts and acutely occluded stents. One potential exception is cardioembolic events to either the brachial artery or the common femoral artery, which can be expeditiously removed with surgical embolectomy and use of local anesthesia, or to the aortic bifurcation, where expeditious revascularization may be important to minimize risks from metabolic systemic complications. Of the previously used thrombolytic agents, streptokinase urokinase and tissue plasminogen activators, only tissue plasminogen activators (t-PAs) are in active use in the United States. A complete discussion of the available pharmacologic lytic agents and their actions is provided in Chapter 36.
Peripheral thrombolytic therapy is administered through a catheter-directed approach to achieve regional thrombus dissolution with minimal systemic fibrinolysis. However, a moderate systemic proteolytic state often results from the use of thrombolytic agents, limiting their use to patients without contraindications to such a state (Box 162-1).6–8
Technique
Most physicians prefer a retrograde contralateral femoral approach when dealing with ALI. We routinely use a micropuncture needle to perform a single wall retrograde puncture of the common femoral artery, with a low threshold for use of ultrasound guidance. Antegrade ipsilateral femoral access carries a higher risk of hemorrhagic complications and increases the risk of wound infection if an open vascular procedure becomes necessary. Once access is obtained, a short 5F sheath is introduced and diagnostic angiography is performed. A complete evaluation of the runoff vessels is obtained before an attempt is made to cross the occlusion with the sheath. This evaluation assists in the recognition of embolic events and also identifies a potential target vessel for bypass in the event of failed thrombolytic therapy. We routinely place a 45-cm or 55-cm 5F sheath over the aortic bifurcation into the contralateral femoral artery to provide support for crossing the occlusion. A straight 4F Glidecath catheter (Terumo Interventional Systems, Somerset, NJ) with a 0.035-inch angled Glidewire (Terumo Interventional Systems) and liberal fluoroscopic “road-mapping” techniques are employed to cross the occlusion. A 0.035-inch Quick Cross Support Catheter (Spectranetics Corporation, Colorado Springs, Colo) may be useful for crossing the occlusion if more support is needed. An alternative is to use a smaller hydrophilic V-18 Control guide wire (Boston Scientific Corporation, Natick, Mass) to cross the occlusion. The “guidewire traversal test”—the ease with which a hydrophilic wire is able to cross the occlusion—is a good predictor of technical success in CDT. However, one must take care to avoid intimal dissection in traversing the occlusion.9
In the treatment of a thrombosed bypass graft, multiple oblique angiographic views may be required to visualize the proximal stump of the graft. If a bypass graft cannot be entered from the native artery, ultrasound-guided direct puncture of the graft may be helpful. Open cut down is required to remove the sheath and repair the graft once thrombolysis is complete. Direct graft puncture is also useful in treating thrombosed axillofemoral grafts. Two separate sheaths are placed directly into the occluded graft about 5 cm apart in crossing fashion so that the sheath tips are directed toward each other. Two separate thrombolysis catheters are then used to cross the occluded graft from opposite directions.
When a single catheter and sheath are used to cross an occlusion, a distal angiogram is performed to confirm the catheter’s location in the true lumen of the distal native vessel. A catheter exchange is performed to place a specifically designed, 0.035-inch compatible infusion catheter across the occluded segment. We prefer to use the Fountain Infusion Catheter (Merit Medical Systems, Inc., South Jordan, Utah) which is a 4F catheter with a working length of 90 or 135 cm and infusion lengths of 5 cm to 50 cm (Fig. 162-1A,B).
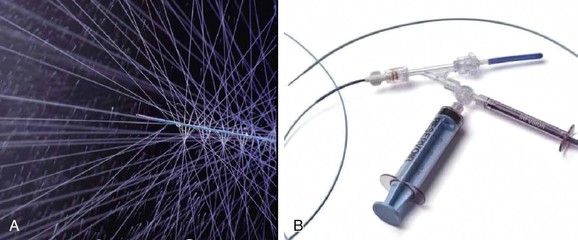
Figure 162-1 A, Distal end of Fountain Infusion Catheter Merit Medical Systems, Inc., South Jordan, Utah), which has multipleside holes, with test infusion demonstrated. B, Proximal end of the Fountain Catheter.
Multiple side holes in this infusion catheter are designed to distribute the lytic agent throughout the thrombus. A ball-valve occlusion wire included with the catheter directs flow out the side holes and temporarily occludes the end of the catheter. The distal side hole marker should be placed at the end of the thrombus, and the proximal side hole located just proximal to the thrombus origin. A list of various infusion catheter sizes and designs is shown in Table 162-2. Both low-dose and high-dose regimens can be used with recombinant t-PA (rt-PA) (Alteplase, Genentech, Inc., South San Francisco). The low-dose regimen prescribes a 1-mg bolus, followed by a continuous infusion of 0.5 to 1 mg/hour. The infusion can run for 10 to 12 hours or overnight with another arteriogram performed the following day. Heparin is administered through the proximal side port of the 5F sheath at 300 to 500 units/hour to prevent peri-sheath thrombosis.
Table 162-2
Therapeutic Infusion Systems
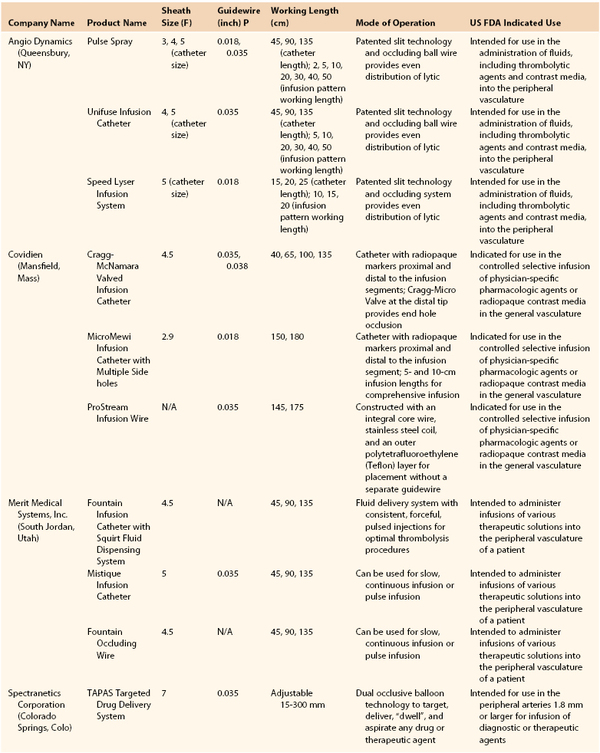
From Endovascular today 2013 buyers guide, 2012; Vol 11: p106. http://bmctoday.net/evtoday/buyersguide/2013.
We currently use a high-dose t-PA regimen for shorter periods. In our experience, this protocol has been very effective with no increase in bleeding complications. This regimen involves giving a 10-mg pulse-spray bolus of t-PA, followed by a continuous infusion at 0.05 mg/kg/hour with a maximum dose of 4 mg/hour.10,11 The high-dose infusion is continued for 6 hours before angiography is again performed. We routinely check the serum fibrinogen level every 4 hours and discontinue the infusion if the fibrinogen level drops below 100 mg/dL. All patients are kept NPO (nothing by mouth) status and at bed rest in a critical care setting so they can be observed for clinical or laboratory evidence of local or systemic bleeding.
Results
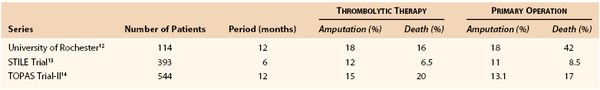
In the 1990s, three multicenter randomized trials were published comparing thrombolysis with operation for arterial occlusion (Table 162-3).12–14
The first trial, the Rochester study, randomly assigned 114 patients with acute limb-threatening ischemia to thrombolysis with urokinase (57 patients) or to immediate operation (57 patients).12 At 1 year, the amputation-free survival rates were 75% and 52%, respectively, a statistically significant difference. A closer analysis revealed this finding to be the result of a higher mortality rate in the operative group caused by perioperative cardiopulmonary complications. It appeared that taking patients with severe limb ischemia directly to operation without the opportunity for preparation resulted in a high frequency of complications that culminated in death.
The second large multicenter evaluation was the Surgery versus Thrombolysis for Ischemia of the Lower Extremity (STILE) trial.13 In this study, 393 patients were randomly assigned to surgery or to thrombolysis with either rt-PA or urokinase. Clinical outcomes for the rt-PA and urokinase groups were similar, so the data were combined for an overall comparison of thrombolysis and surgery. Post hoc stratification of patients into two subgroups on the basis of duration of symptoms before enrollment (more or less than 14 days) showed that among patients with symptoms of longer duration, the surgical group had lower amputation rates than the thrombolysis group at 6 months (3% vs. 12%). In contrast, among patients with symptoms of shorter duration, patients assigned to thrombolysis had lower amputation rates than surgical patients (11% vs. 30%).
The third multicenter trial to evaluate thrombolytic therapy was the Thrombolysis or Peripheral Arterial Surgery (TOPAS) trial.14 Recombinant urokinase (r-UK) therapy (4000 IU/min for 4 hours, followed by 2000 IU/min) was compared with primary operation in 544 patients with lower extremity native artery or bypass graft occlusions of 14 days’ duration or less. There was no significant difference in amputation-free survival rates or mortality rates between the two groups at the time of discharge from the hospital. Likewise, the amputation-free survival rates 6 months after randomization were not significantly different: 71.8% in the r-UK group and 74.8% in the operative group. At the end of 6 months, 31.5% of the patients in the r-UK group had avoided amputation or death without the need for anything more than a percutaneous procedure. By contrast, 94.2% of the patients who had had primary operation underwent open surgery, a rate that was not unexpected owing to the design of the trial. The median length of hospitalization was 10 days in both treatment groups. Among the patients assigned to thrombolysis, those with occlusions in bypass grafts had better clinical outcomes, better rates of clot dissolution, and lower rates of major hemorrhagic complications than did those with native artery occlusions.
In patients with acute limb ischemia, CDT leads to resolution of thrombus with satisfactory clinical results in 75% to 92% of patients.1 The factors that portend a higher likelihood of success are: (1) recent graft occlusion (less than 14 days), (2) guidewire traversal of the occluded graft, (3) graft patency of at least 1 year prior to the thrombotic event, and (4) the presence of a remediable lesion that may be treated. Factors associated with a poor outcome after thrombolysis include diabetes, current smoking, and prosthetic graft material. The reported 24-month vessel patency rate after thrombolysis for native arterial occlusions was 79% if an underlying lesion was identified and treated compared with 9.8% when no lesion was identified. For bypass graft thrombotic events, post-thrombolysis graft patency rates are between 10% and 40% at 2 years, making a case for replacement of a failed graft, especially if an anatomic lesion is not identified as an etiology of graft thrombosis.
Complications
Hemorrhagic complications are the primary cause of morbidity following CDT. In the TOPAS trial, major hemorrhagic complications occurred in 32 patients (12.5%) in the r-UK group, compared with 14 patients (5.5%) in the surgery group.14 Patient age, duration of infusion, and activated partial thromboplastin time at baseline were unrelated to the risk of bleeding. Intracranial hemorrhage occurred in 4 patients in the r-UK group (1.6%), 1 of whom died. There were no instances of intracranial hemorrhage in the surgery group. The risk of bleeding was significantly greater when therapeutic heparin was used. In 102 patients who received therapeutic heparin, bleeding occurred in 19 patients (19%). By contrast, of the 150 patients in whom therapeutic heparin was not used, bleeding occurred in only 13 (9%). These data are the basis for the use of low-dose heparin infusions via the sheath, rather than therapeutic heparin dosing, in current protocols. Other complications include compartment syndrome after reperfusion, in 5% to 25% of patients, and acute renal insufficiency from rhabdomyolysis, occurring in up to 20% of patients.
Although not widely reported, distal embolization during CDT is common. Symptoms may worsen, or a distal Doppler ultrasound signal may be lost during the initial phase of lytic infusion. This is often managed by continued treatment with the thrombolytic agent and pain medication. More significant distal embolization that does not resolve in a few hours requires immediate arteriography, with the occasional need to reposition the catheter to a more distal location. The use of glycoprotein IIb/IIa inhibitors has been proposed as a mechanism to reduce distal embolization but does not appear to improve outcomes.15
Pharmacomechanical Thrombectomy
Several of the mechanical clot removal devices are intended to be used in conjunction with thrombolytic agents. Mechanical mixing (Trellis catheter), ultrasound energy (EKOS and OmniSonics), or “power pulse injection” (AngioJet) is used to accelerate the speed of thrombolysis. The resultant decrease in the dose of lytic agents and duration of thrombolysis has the potential to minimize the bleeding risk associated with standard CDT and to shorten the time to reperfusion.
Trellis Catheter.
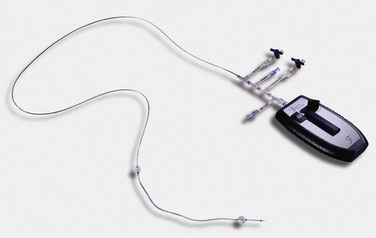
The Trellis Peripheral Infusion System (Bacchus Vascular, Inc., Santa Clara, Calif) is an 8F coaxial system that can be passed over a 0.035-inch guide wire. The proximal portion of the device, which remains outside the patient, has five separate entry ports (Fig. 162-2). Two of these ports are used to inflate the compliant balloons located at each end of the infusion and dispersion segment. The balloons can be inflated to a maximum diameter of 14 mm. When inflated, the balloons are designed to isolate the treatment zone and keep the thrombolytic agent locally at the infused concentration. The balloons also help prevent downstream and upstream release of embolic debris. After balloon isolation of the treatment zone, the thrombolytic agent is infused through a separate proximal infusion port. A fourth, separate guide wire flush port facilitates the performance of distal arteriography while the device is in place or may be used for the distal infusion of thrombolytic agents. The proximal end hole accommodates the guide wire or the dispersion wire through the central lumen.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree