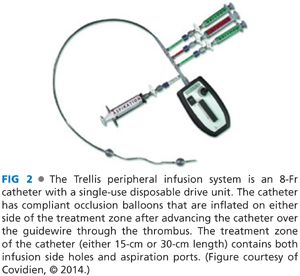
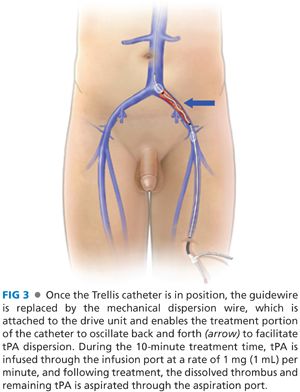
■ The Trellis system is composed of an infusion catheter of either 15- or 30-cm infusion length, with compliant occlusion balloons at either end of the infusion ports (FIG 2). Following placement over the guidewire, the end occlusion balloons are inflated in order to isolate the area of planned pharmacomechanical thrombolysis (FIG 3). A sinusoidal dispersion wire is then advanced through the core of the catheter and attached to a disposable drive unit, which when activated uses mechanical forces to disperse the tPA through the thrombus. After an infusion of 6 mg of tPA over the span of 10 minutes, aspiration of thrombus and debris is performed from the treated segment. The occlusion balloons concentrate tPA within the treatment segment, enabling multiple infusion and dispersal sessions during the same procedure with minimal systemic delivery of thrombolytic agent (FIG 4).

Stenting of Underlying Venous Stenoses or Venous Compression Syndromes
■ Following clearance of acute thrombus from the iliofemoral system, underlying venous lesions that provoked DVT formation, or focal external compression may become apparent on completion phlebography. These lesions should be addressed during the same treatment session to minimize the risk of recurrence. IVUS may be particularly useful in this regard.
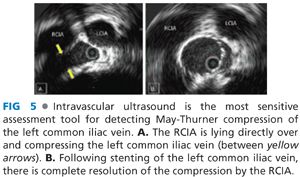
■ Although fixed stenoses may occur throughout the venous system, the most common location for extrinsic compression occurs at the point where the left common iliac vein passes beneath the overlying right common iliac artery (RCIA) (FIG 5). After recognizing this compression and successful removal of thrombus proximal or distal to this lesion, the stenosis may be safely resolved with stenting (FIG 6). This is best performed by upsizing the interventional sheath to at least 10 Fr followed by deployment of a self-expanding, braided, stainless steel Wallstent (Boston Scientific, Watertown, MA). In conjunction with completion venography, IVUS is then used to quantify the extent of residual compression.
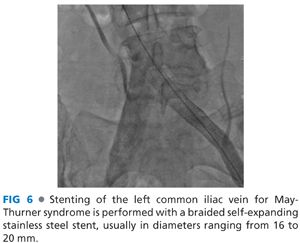
■ Stent diameter is chosen based on IVUS-obtained measurements, but diameters commonly chosen for common iliac vein placement in May-Thurner patients range from 16 to 20 mm. In this application, it is important to choose longer stents that provide additional surface apposition in the common or even external iliac veins to present stent dislodgement and migration. Wallstents are particularly appropriate in this regard as they will shorten or extend in proportion to the ultimate treatment diameter and feature exposed wires at either end to optimize vein wall engagement.
■ Once appropriately sited and deployed, poststent dilation is necessary to ensure optimal deployment and migration resistance. Some discomfort will be experienced by the “awake” patient during these procedures, and stenting molding should be guided by patient tolerance under these circumstances.
Completion Imaging
■ Completion phlebography documents resolution of target stenosis and reciprocal reduction in collateral venous flow.
■ The presence of persistent collaterals suggests residual venous stenosis or compression; IVUS should be reperformed in this circumstance to confirm wall apposition and stent expansion. Repeat balloon dilation may be necessary in these circumstances until sufficient expansion is achieved.
Closure of the Femoral Vein Access
■ Following sheath removal, manual pressure is held over the venous puncture site. Closure devices are not appropriate or indicated for management of venous access.
■ Patients need to remain supine for at least 1 hour following sheath removal.
■ Therapeutic intravenous anticoagulation with unfractionated heparin is initiated at the completion of the procedure. Maintenance of full anticoagulation without interruption throughout the early postoperative period is imperative to procedural success.
OPERATIVE MANAGEMENT OF ILIOFEMORAL DEEP VEIN THROMBOSIS
Iliac Venous Thrombectomy
■ For most clinical scenarios, open venous thrombectomy has largely been supplanted by the interventional, image-guided techniques described in the preceding sections. In patients with limb-threatening phlegmasia cerulean dolens or those with contraindications to lytic therapy or contrast administration, open surgical thrombectomy remains an effective and necessary treatment modality.
■ Whenever possible, surgical thrombectomy is performed under general anesthesia with positive pressure ventilation to reduce the risk of intraoperative PE.
■ A vertical inguinal incision is made to allow exposure and control of the CFV, femoral vein, saphenofemoral junction, and the profunda femoris vein. Once these venous structures have exposed, the patient is systemically anticoagulated with 100 units/kg of intravenous heparin.
■ A longitudinal venotomy is made in the CFV, and a no. 8 or no. 10 venous thrombectomy catheter is then passed up to the level of the common iliac vein and thrombectomy is performed. Attempts are made to clear the majority of the iliac thrombus before passing the thrombectomy catheter into the vena cava in order to reduce the likelihood of pulmonary embolization.
■ Back-bleeding may not be present due to competent iliac vein valves, or back-bleeding can occur from the hypogastric vein even without clearance of the thrombus within the common iliac vein. Therefore, back-bleeding should not be used as an indicator of effective thrombus clearance and venography should be performed as a routine after completion of iliac and infrainguinal thrombectomy.
Infrainguinal Femoral Venous Thrombectomy
■ Following iliac venous thrombectomy, heparinized saline should be used to flush the iliac vein, the proximal external iliac vein (or distal CFV) should be clamped, and then any thrombus at the proximal (peripheral) aspect of the venotomy should be extracted with forceps.
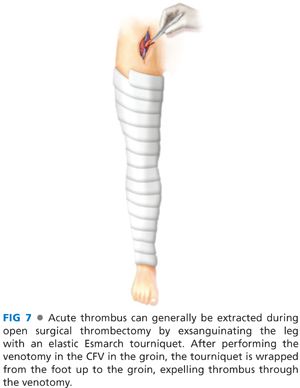
■ Infrainguinal thrombus can then be removed by manual massage or by exsanguinating the leg with an Esmarch bandage, sequentially applied from the foot to the groin, with sufficient overlap to provide continuous compression (FIG 7). Clot is delivered through the venotomy at the groin.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


