Acute Bronchitis and Community-Acquired Pneumonia
ACUTE BRONCHITIS
Acute bronchitis is an inflammation of the tracheobronchial tree, usually in association with a generalized respiratory infection affecting 40/1000 adults each year in the United Kingdom.1 It is the fifth most common diagnosis in patients presenting with cough.2 It occurs most commonly during the winter months and is associated with respiratory viruses, including rhinovirus, coronavirus, influenza viruses, and adenovirus. Mycoplasma pneumoniae, Chlamydia pneumoniae, and Bordetella pertussis may also cause bronchitis. Secondary invasion with bacteria such as Haemophilus influenzae and Streptococcus pneumoniae may also play a role in acute bronchitis. However in only 50% of persons with acute bronchitis have a pathogen identified.1
Cough is the most prominent manifestation of acute bronchitis. Initially, the cough is nonproductive, but later mucoid sputum is produced. Still later in the course of the illness, purulent sputum is present. Many patients with acute bronchitis also have tracheitis. Symptoms of tracheal involvement include burning substernal pain associated with respiration and a very painful substernal sensation with coughing. Rhonchi and coarse crackles may be heard on examination of the chest; however, there are no signs of consolidation and the chest radiograph shows no opacity. Cough persists on average 11.4 days.1,2
Most cases of acute bronchitis require measures directed only at relieving cough. There are very few good clinical trials comparing various symptomatic relief measures to placebo so we do not know whether or not they are better than placebo.1 Such measures include analgesics, antihistamines, antitussives, inhaled or oral beta2-agonists, expectorants and mucolytics. For patients with fever or a predominant tracheitis component and purulent sputum, the sputum should be gram stained and cultured. If there is a predominant microorganism seen in the presence of more than 25 polymorphonuclear neutrophils and fewer than 10 squamous epithelial cells per low-power field, antibiotic therapy directed against S. pneumoniae and H. influenzae should be instituted. Most patients, however, do not require antibiotic therapy for acute bronchitis; it is a self-limited disease. Indeed, overuse of antibiotics in this setting is a driver of antimicrobial resistance.
COMMUNITY-ACQUIRED PNEUMONIA: EPIDEMIOLOGY, CLINICAL ASSESSMENT, AND DIAGNOSTIC WORK-UP
Pneumonia is defined as inflammation and consolidation of lung tissue due to an infectious agent (see also Chapter 122). Pneumonia that develops outside the hospital is considered community-acquired pneumonia (CAP). A clinical definition of pneumonia is two or more of the following symptoms/physical findings: productive cough, purulent sputum, dyspnea or tachypnea (respiratory rate >20 breaths per minute), rigors or chills, pleuritic chest pain in conjunction with a new opacity on chest radiograph.3 Pneumonia developing 72 hours or more after admission to hospital is nosocomial, or hospital acquired. There is still some debate as to whether nursing home–acquired pneumonia (NHAP) should be considered community-acquired or nosocomial pneumonia. In recent years, the concept of healthcare-associated pneumonia (HCAP) has arisen. This term was introduced in 2005 to recognize that as a result of the shift of many inpatient hospital services to home, ambulatory care facilities and extended care facilities patients were at risk for infections that resembled hospital-acquired infections.4 HCAP was defined as pneumonia in patients who have been hospitalized in an acute-care hospital for 2 or more days in the past 90 days, have been residents in a nursing home or long-term care facility (nursing home–acquired pneumonia, or NHAP), received intravenous antibiotic therapy, chemotherapy, or wound care within the past 30 days, or attended a hospital or hemodialysis clinic.5
This chapter focuses on CAP.
 EPIDEMIOLOGY
EPIDEMIOLOGY
CAP is both a common and serious illness. In combination with influenza, it is the most frequent cause of infection-related death and the eighth leading cause of death overall in the United States.6,7 CAP occurs in approximately 4 million adults in the United States—accounting for 10 million physician visits, 1.1 million hospitalizations, and 50,000 deaths per year.8–10 It accounts for ~3.5 million deaths each year globally and is the highest cause of mortality among infectious diseases.11 Changes in the epidemiology (both emergence of new pathogens and changing antimicrobial susceptibility of old ones) of the various pathogens, difficulty in making an etiologic diagnosis and complex guidelines for management make this illness a challenge for both patients and their physicians.
The overall attack rate is about 12 cases per 1000 persons per year. In adults, the rate of admission to hospital for the treatment of pneumonia is low from age 17 to 55 years, at which point it begins to increase. The attack rates are highest at the extremes of age.
The epidemiology of pneumonia has changed in recent years. This is due in part to changes in the population at risk and in part to the discovery of new microbial agents that cause pneumonia and changes in antimicrobial susceptibility of old microbial agents, such as S. pneumoniae, H. influenzae, and Staphylococcus aureus. Population changes include continued increase in the number and proportion of patients who are 65 years of age or older.
There has been a steady increase in the number of organ transplant recipients in the general population and in the number of patients with HIV infection. This has created a subset of patients with CAP who may be infected not only with the traditional pathogens that cause pneumonia but also with opportunistic pathogens; furthermore, these patients may have severe or atypical presentations of this infection. Newer pathogens recognized as causing pneumonia include Hantavirus, SARS CoV, human metapneumovirus, S. aureus isolates carrying the Panton-Valentine leukocidin (PVL) genes, and methicillin-resistant S. aureus (MRSA). Pneumocystis jiroveci, previously a rare cause of pneumonia in intentionally immunocompromised patients, is a common cause of pneumonia in HIV-infected patients with CD4 counts of less than 200/μL.
In a study carried out in a Swedish town in which all persons 60 years of age and older were studied, independent risk factors for CAP were alcoholism, relative risk (RR) 9; asthma, RR 4.2; immunosuppression, RR 1.9; age greater than 70 years versus age 60 to 69 years, RR 1.5.
Risk factors for specific etiologies of pneumonia may differ from those for pneumonia as a whole. Thus, dementia, seizures, congestive heart failure, cerebrovascular disease, and chronic obstructive lung disease were risk factors for pneumococcal pneumonia in one study. In other studies, cigarette smoking and asthma have been found to be independent risk factors for invasive pneumococcal disease. Among HIV-infected patients, the rate of pneumococcal pneumonia is 41.8 times higher than those in the same age group who are not HIV infected. However, with the advent of highly active antiretroviral therapy, the incidence of pneumococcal bacteremia among HIV-infected persons has dropped from 24.1 episodes per 1000 patient-years to 8.2 per 1000 patient-years. Up until a recent study from the Centers for Disease Control and Prevention, the effect of chronic illness on the incidence of invasive pneumococcal disease in adults was underappreciated. In this study, the overall incidence rates of invasive pneumococcal disease was 8.8/100,000 adults. For those with diabetes it was 51.4; 62.9 for adults with chronic lung disease; 93.7 for those with chronic heart disease; and 100.4 among those who abused alcohol. The rate was highest in adults with solid cancer, 300.4, and HIV/AIDS, 422.9.
Risk factors for Legionnaires’ disease include male gender, tobacco smoking, diabetes, hematologic malignancy, cancer, end-stage renal disease, and HIV infection. Risk factors for severe respiratory syncytial virus infection in elderly persons include the presence of underlying chronic pulmonary disease (odds ratio [OR] 3.97), functional disability (OR 1.67), and low serum neutralizing antibody titer (OR 5.89). The usual risk factors for aspiration pneumonia are altered level of consciousness and various neurologic diseases that interfere with the swallowing mechanism. Recently, there has been an association between the use of gastric acid suppressive drugs and aspiration pneumonia. The incidence rates of pneumonia in non–acid-suppressive drug users and those who used these agents was 0.6 and 2.45 per 100 person-years, respectively. The risk seemed to be highest among those using proton pump inhibitors.
There is seasonal variation in the rate of pneumonia. Both attack rates and mortality rates are highest in the winter months. This is likely due to many factors, including more time spent indoors (crowding) and hence more opportunity for person-to-person spread of infectious agents. In a study carried out in Tennessee, the weekly frequency of invasive pneumococcal disease correlated with the weekly frequency of isolation of respiratory syncytial virus and influenza virus.
Antimicrobial resistance of the common bacterial pathogens is also a key component of the epidemiology of CAP. Penicillin-resistant Staphylococcal pneumoniae (PRSP) is now a fact of life in most North American communities. Many of the PRSP isolates are resistant to three or more antibiotic classes (multidrug resistance). In one study, 14% of bacteremic S. pneumoniae isolates were resistant to penicillin, 12% to ceftazidime, and 24% to trimethoprim–sulfamethoxazole. In a recent study, the investigators examined 1817 S. pneumoniae isolates collected from patients with community-acquired respiratory tract infections at 44 US medical centers during the winter of 2002 to 2003. The overall rates of resistance were as follows: penicillin 34.2%; ceftriaxone 6.9%; erythromycin 29.5%; clindamycin 9.4%; tetracycline 16.2%; and trimethoprim– sulfamethoxazole 31.9%. There was no resistance to the following agents: vancomycin, linezolid, and telithromycin. Multidrug resistance was present in 22.2% of the isolates and 2.3% of the isolates had ciprofloxacin MICs of greater than or equal to 4 μg/mL. Currently in the United States about 26% of S. pneumoniae are resistant to penicillin and 25% are resistant to erythromycin.12 However within serotype nonsusceptibility varies widely across the various states.12 Serotypes 6C, 15A, 19A, 23A account for most of the penicillin nonsusecptibility and 6C, 15A, 19A, and 22F account for most of the isolates that are both penicillin and erythromycin nonsusceptible.12 Fortunately, it is possible to predict who is likely to have pneumonia due to PRSP. Previous use of beta-lactam antibiotics, alcoholism, noninvasive pneumococcal disease, age less than 5 or greater than 65 years, and immunosuppression are risk factors for PRSP pneumonia. In Canada, S. pneumoniae resistance to levofloxacin remains very low, peaking at 1.5% in 2008, while no isolates tested in 2011 were resistant.13 However in Spain 3.3% of S. pneumoniae isolates are resistant to levofloxacin and there is evidence of clonal spread.14
 CLINICAL MANIFESTATIONS
CLINICAL MANIFESTATIONS
Symptoms that are suggestive of pneumonia include fever, chills, pleuritic chest pain, and cough. The cough may be nonproductive (dry) or productive of mucoid or purulent sputum. It may be rusty in color and frankly bloody; in patients with a lung abscess (anaerobic infection), it may have a foul odor. The latter is suggestive of anaerobic infection.
Elderly patients complain of fewer symptoms than do younger patients. Indeed, those greater than 75 years of age with pneumonia had 3.3 fewer total symptoms than did patients aged 18 to 44 years with pneumonia.
For some time it was held that typical pneumonia (due to pyogenic organisms such as pneumococcus, staphylococcus, or H. influenzae) could be distinguished from that due to M. pneumoniae, Legionella spp., and C. pneumonia – so-called atypical pneumonia agents – on the basis of a distinct clinical presentation. Atypical pneumonia is said to be characterized by a more indolent illness than that of typical pneumonia, with a cough that is nonproductive or productive of mucoid sputum only. Careful studies have shown that one cannot reliably distinguish between typical versus atypical pneumonia on clinical grounds. However, this is not to say that a careful history and physical examination are not helpful in suggesting a cause of the pneumonia. Table 128-1 gives a partial list of clues to the cause of pneumonia that may be obtained from the history and physical examination.
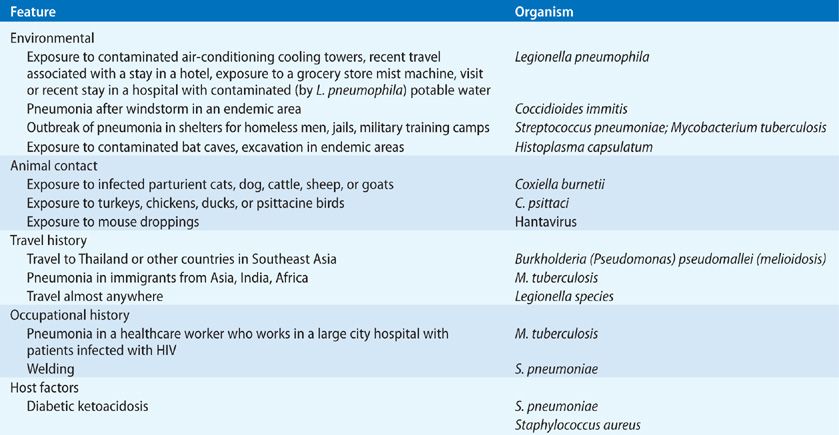
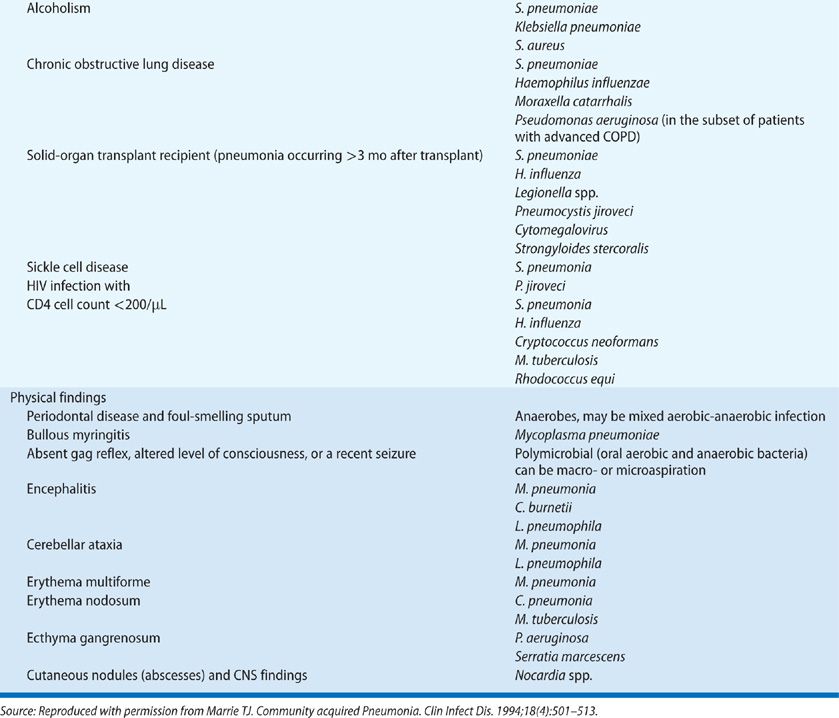
Nonrespiratory symptoms such as headache, nausea, vomiting, abdominal pain, diarrhea, myalgia, and arthralgia are also common symptoms in patients with pneumonia. It is wise to remember that the elderly complain of fewer symptoms with pneumonia than do younger patients.
In some instances extrapulmonary signs and symptoms may dominate the clinical picture. Thus, M. pneumoniae may be complicated by a variety of neurologic manifestations including encephalitis, meningitis, and cranial nerve palsies. In addition a maculopapular skin rash is not uncommon. Occasionally, Stevens–Johnson syndrome develops. Patients with Legionella pneumonia may have glomerulonephritis or cerebellar ataxia. One should also remember that pyogenic bacteria that cause pneumonia (S. aureus, S. pneumoniae) can cause metastatic infections such as endocarditis, brain abscess, and meningitis. Indeed, all patients with pneumonia and S. aureus bacteremia should have a careful evaluation for endocarditis.
 PHYSICAL EXAMINATION
PHYSICAL EXAMINATION
Fever is usually present, but some patients may be hypothermic (a poor prognostic sign), and some (20%) are afebrile at the time of presentation with pneumonia. Crackles are heard on auscultation over the affected area of lung, and physical findings of consolidation (dullness to percussion, increased tactile, vocal fremitus, whispering pectoriloquy, and bronchial breath sounds) are present in about 20% of patients with pneumonia. A pleural friction rub is heard in about 10% of cases. Early in the course of the illness physical examination of the chest may be normal. It is most important to count the respiratory rate for at least 1 minute in patients suspected of having pneumonia. A respiratory rate of ≥28 breaths per minute in an adult is indicative of severe pneumonia and an alert that such patients are at high risk for respiratory failure.
 RADIOGRAPHIC DIAGNOSIS
RADIOGRAPHIC DIAGNOSIS
A clinical suspicion of pneumonia usually prompts a chest radiograph. An opacity on the chest radiograph is considered the gold standard for the diagnosis of pneumonia. However, this opacity may be due to infection, infarction, hemorrhage, edema fluid, malignancy, or inflammation caused by a variety of processes, such as vasculitis or adverse drug reactions. Several studies have shown that radiologists cannot differentiate bacterial from nonbacterial pneumonia on the basis of the radiograph. Representative chest radiographs of patients with pneumonia are shown in Figures 128-1 to 128-4. For patients with pneumonia treated on an ambulatory basis, there is considerable disagreement (in up to 50% of cases) between the radiologist’s reading of the chest radiograph regarding the presence of pneumonia compared with that of the attending physician. In about 20% of patients with symptoms compatible with pneumonia and a plain chest radiograph read as normal or no pneumonia by the radiologist, computed tomography of the chest will be compatible with pneumonia. It is noteworthy that when patients who are admitted to hospital with a clinical diagnosis of pneumonia (radiologist says no pneumonia) are compared with those with radiologist-confirmed pneumonia, there is no difference in mortality between the two groups. While the percentage of patients with positive blood cultures does not differ between the groups, the microorganisms isolated do; about 60% of the isolates for those with definite pneumonia are S. pneumoniae compared with 31% for those with clinical pneumonia.
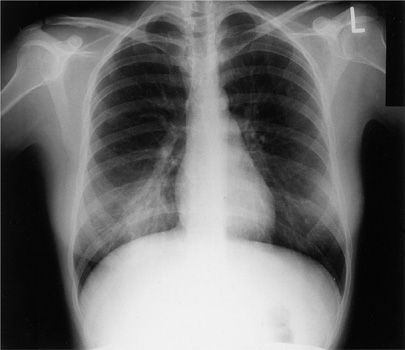
Figure 128-1 Right lower lobe pneumonia due to Coxiella burnetii (Q fever). This young woman developed pneumonia after exposure to the products of conception of her infected pet cat.
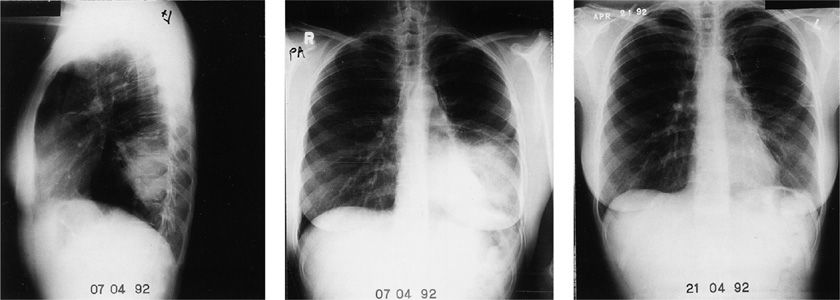
Figure 128-2 Serial chest radiographs of a 32-year-old nurse with Chlamydia psittaci pneumonia. She was severely ill with fever, chills, and headaches. She had severe fatigue for 8 months after this episode of pneumonia.
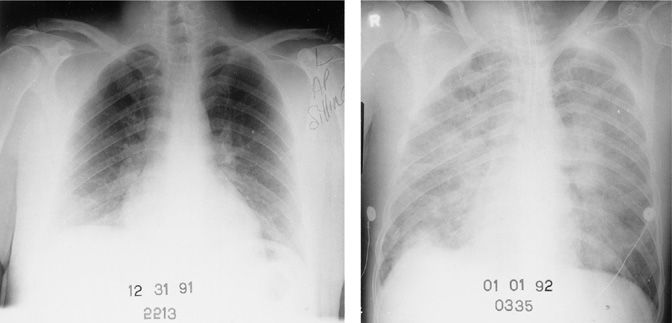
Figure 128-3 Chest radiographs showing rapidly progressive diffuse pulmonary opacities in a 22-year-old man with bacteremic Streptococcus pneumoniae pneumonia. This patient had had his spleen removed 6 years earlier. He rapidly developed septic shock and died about 8 hours after admission.
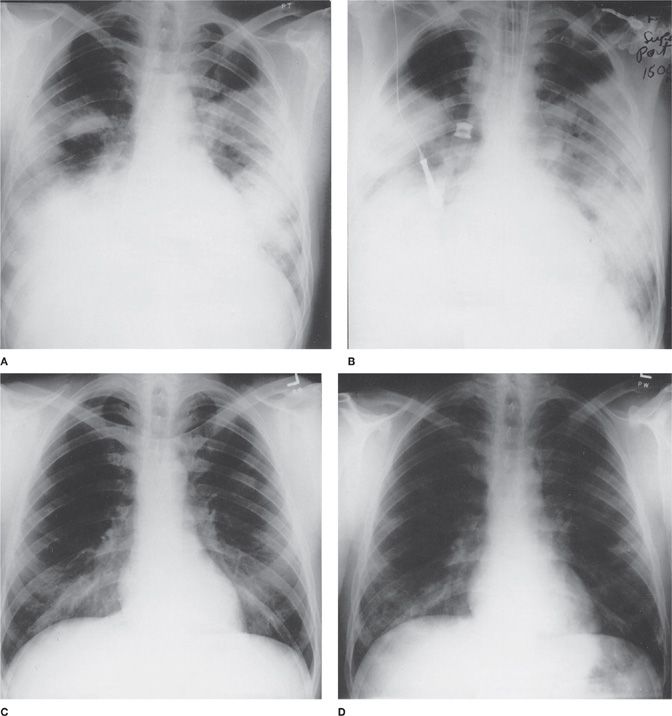
Figure 128-4 Serial chest radiographs (temporal sequence: A., B., C., D.) of a 40-year-old man with pneumonia due to Legionella pneumophila serogroup 6.
 ETIOLOGIC DIAGNOSIS
ETIOLOGIC DIAGNOSIS
Pneumonia represents a difficult challenge for the clinician, since the etiology cannot be determined from the clinical presentation and data from microbiologic studies are not available for at least 48 hours. Even then, in the case of microorganisms isolated from the sputum, one cannot be sure that this is the organism causing the pneumonia, and not just a microorganism that had colonized the upper airway through which the sputum passed on its way to the specimen jar. For this reason, it is useful to categorize the etiology of pneumonia as definite or probable (Table 128-2).
Source: Data from Fang GD, Fine M, Odoff J, et al. New and emerging etiologies for community-acquired pneumonia with implications for therapy: A prospective multicenter study of 359 cases. Medicine. 1990;69(5):307–316.
Newer tests that can be used to help make an etiologic diagnosis of pneumonia include testing urine for antigens of S. pneumoniae or Legionella. However, only tests for Legionella pneumophila serogroup 1 are readily available. Nasopharyngeal swabs can be test for a variety of pathogens using multiplex polymerase chain reaction.
The etiology of CAP as determined in prospective studies is given in Tables 128-3 to 128-5. Table 128-4 shows data for patients with severe pneumonia requiring admission to intensive care units. Table 128-5 gives the etiologic data for bacterial pneumonia in patients with HIV infection. Early in the course of the HIV epidemic, P. jiroveci accounted for most cases of pneumonia. Now, with widespread use of prophylaxis to prevent Pneumocystis pneumonia, bacterial pneumonia is more common in HIV disease than previously seen (see Chapter 123). Indeed, the rates of pneumococcal pneumonia and H. influenzae pneumonia are 20 times higher among HIV-infected persons than in those of an age- and sex-matched population without HIV infection. In any young person with pneumococcal bacteremia, consider underlying HIV infection. However, one should not forget about P. jiroveci pneumonia, since many persons do not know they have HIV and this form of pneumonia is the presenting manifestation of HIV disease in these individuals. Likewise, one should never forget Mycobacterium tuberculosis as a cause of pneumonia, especially in the elderly.
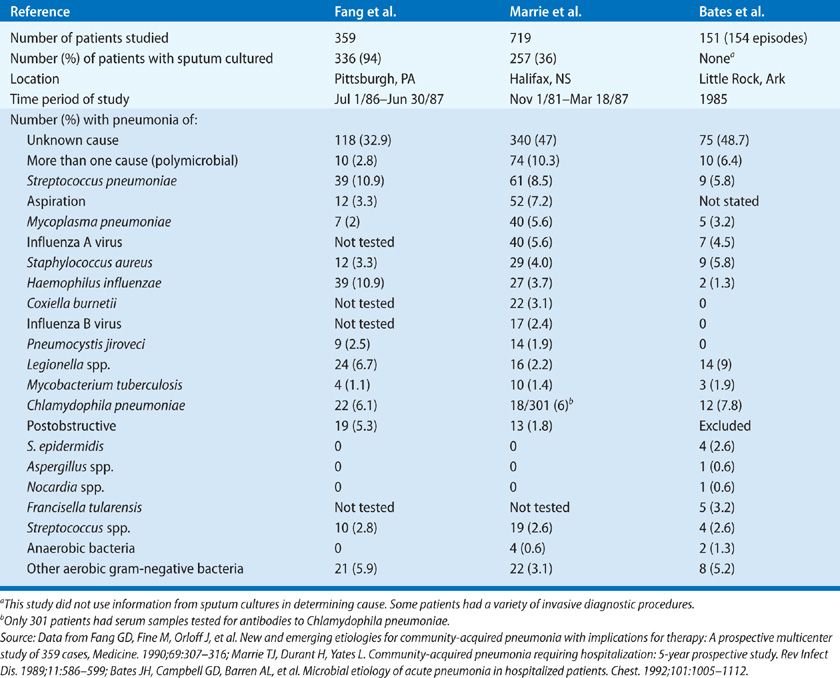
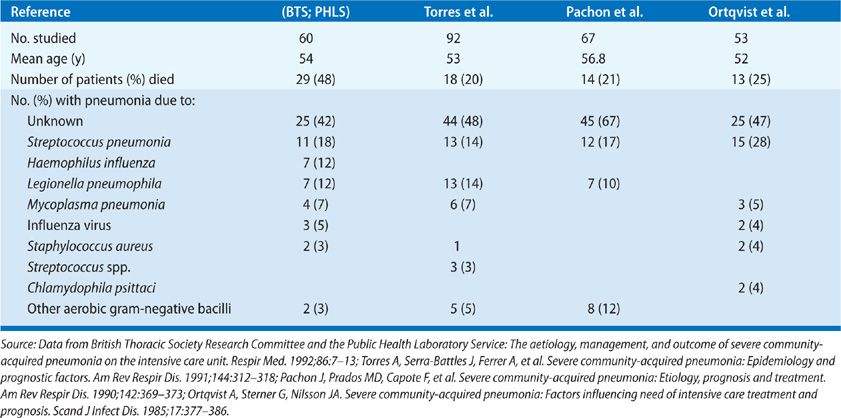
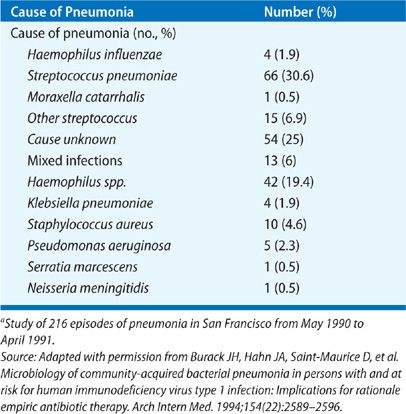
While the data given in the tables are old, these studies remain some of the best that have been done to determine the etiology of pneumonia. In a study carried out at a Veterans Administration Hospital in Houston, Texas during 2011 and 2012, using the most up to date methods, no etiology was found in 45.9%; 23% bacterial cause; 16% viral (about 20% of these had a coinfection with a bacterial pathogen); 26% had a syndrome indistinguishable from bacterial pneumonia and 17% were clearly uninfected.15
Given the difficulty in making an etiologic diagnosis investigators have sought a biomarker that would distinguish bacterial infection from viral infection or noninfectious causes. Procalcitonin is the biomarker that has been most intensively studied. Procalcitonin is the hormone precursor of calcitonin expressed by C cells of the thyroid gland. The rationale for measuring procalcitonin is that its conversion to calcitonin is inhibited by a number of cytokine and bacterial endotoxins, hence in bacterial infections procalcitonin levels will be high. Indeed a number of studies have shown that serum procalcitonin levels rise and fall rapidly in bacterial infections. A level of 0.25 ng/mL or higher in the setting of pneumonia is felt to be predictive of a bacterial infection as the cause.16 While a number of studies to date have validated the usefulness of procalcitonin as an aid to starting or stopping antimicrobial therapy, it is only that an aid.
 ADMISSION DECISION
ADMISSION DECISION
Once a diagnosis of pneumonia has been made, the next decision is whether or not to admit the patient to the hospital. Now, more than ever, there is considerable pressure to treat as many patients as possible at home. In order to do this, it is important to know the factors that are predictive of complicated course in pneumonia, some of which are given in Table 128-6. Several clinical rules that predict mortality have been developed that are often used to guide the admission decision. Two of these are the pneumonia severity index, often known as the patient outcomes research team (PORT) score (Tables 116-7 and 116-8) and the CURB-65 (confusion; urea; respiratory rate; blood pressure; age >65 years) rule (Table 116-9).17,18 The former, developed by Fine et al.,17 assigns points to each of 20 different items that had been shown to be associated with mortality (see Table 128-7). This system allows categorization of patients with pneumonia into five strata, with increasing risk for mortality from risk classes I to V. Mortality is less than 1% for patients in risk classes I to III, but increases to 9% in class IV, and to 27% in class V (see Table 128-8). Patients in risk classes I and II can usually be treated at home; those in risk class III may require a period of observation in the emergency room before a decision is made about the optimal site of treatment. Patients with pneumonia generally prefer to be treated at home if it can be done safely. As more experience has been gained with this prediction rule, not unexpectedly we have learned that the rule is only a guide. It does not factor social circumstances into the score and since the score is heavily age dependent, many young patients fall into the first three classes when it is readily apparent that they should be admitted.