Chapter 38 Acute and Chronic Aortic Dissection
Medical Management, Surgical Management, Endovascular Management, and Results
Aortic dissection is one of the most complex clinical conditions encountered by vascular surgeons and is associated with significant morbidity and mortality in patients presenting with this disease. The first descriptions of aortic dissection occurred in the sixteenth century and were described in the autopsy of King George II of England in 1761.1 First termed aneurisme dissequant by Laennec in 1819, arterial dissection occurs when a tear in the intimal layer of the vessel leads to separation of wall layers allowing blood to enter between two distinct channels. The newly formed channel layer is termed the false lumen and is separated from the true lumen by a septum composed of the aortic intimal and medial layers. Antegrade blood flow extends into the false lumen distally and may extend to aortic branches (arch, visceral, lower extremity), causing significant end-organ ischemia.
Classification
Anatomic Classification
Two distinct classification systems are currently used to classify aortic dissection based on the location of the intimal tear and the degree of distal extension (Figure 38-1). The DeBakey classification, first developed in 1965, classifies aortic dissection into four types:
• Type I: The entry tear originates in the ascending aorta and extends from the aortic arch into the descending or abdominal aorta for varying distances.
• Type II: The dissection originates and is confined to the ascending aorta.
• Type IIIa: The entry tear is just distal to the left subclavian artery, and the extent is limited to the descending thoracic aorta.
• Type IIIb: The dissection originates just distal to the left subclavian artery and extends for a variable distance of the abdominal aorta.

FIGURE 38-1 The DeBakey and Stanford classification systems for aortic dissection.
(With permission from Erbel R, Alfonso F, Boileau C, et al: Diagnosis and management of aortic dissection. Eur Heart J 22:1642–1681, 2001.)
• A Stanford type A dissection originates in the ascending aorta and encompasses DeBakey types I and II.
• A Stanford type B dissection originates in the descending aorta and includes DeBakey types IIIa and IIIb.
Incidence and Survival Rates of Aortic Dissection
The annual age- and sex-adjusted incidence of acute aortic dissection has been estimated at 2.9 to 3.5 per 100,000 persons in modern series.1,2 It occurs more commonly in men (4 : 1 male-to-female ratio) and type A dissections are more common than type B dissections.1 Dissections are seen most frequently in the 40- to 70-year age range; however, patients with collagen vascular disorders (e.g., Ehlers-Danlos syndrome, Marfan syndrome) are usually seen at a much younger age.3,4
The mortality associated with acute aortic dissection is high and was estimated at 30% in the first 24 hours, 50% by 48 hours, and 90% at 1 year in a classic study by Hirst and colleagues.4 Surgical mortality for acute type A dissections has been found to be 25.1% in a recent review of a population-based International Registry of Acute Aortic Dissection (IRAD) registry.5 Operative mortality in patients with acute, complicated type B dissection approach 25% to 50%.6 Despite the development of improved medical therapy regimens, early mortality for patients with acute type B aortic dissection ranges from 10% to 12%.7
Risk Factors
The most common risk factors for aortic dissection include advanced age, hypertension, and structural abnormalities of the aortic wall. More than 70% of patients are hypertensive. In a study by Juvonen and colleagues, older age, chronic obstructive pulmonary disease, and elevated mean blood pressures were unequivocally associated with aortic rupture following type B dissection.8 Associated structural abnormalities of the aortic wall include: bicuspid aortic valve, aortic coarctation, and chromosomal abnormalities including Turner syndrome and Noonan syndrome. Hereditary conditions associated with collagen vascular disease are also risk factors (e.g., Ehlers-Danlos syndrome, Marfan syndrome). Marfan syndrome is the most common associated disorder in patients younger than 40 years with acute aortic dissection. In patients with Marfan syndrome, a mutation in the fibrillin gene leads to an alteration of the extracellular matrix in connective tissue, resulting in aortic dilatation and dissection.9 Pregnancy, especially with accompanying preeclampsia and hypertension, has also been shown to be an independent risk factor for this condition.10 Cocaine and methamphetamine abuse are also associated with aortic dissection, especially in hypertensive male smokers.11–13
Seasonal variation has been observed in the development of aortic dissection with more patients being seen in the winter months.14 It is also more likely to occur during the hours of 6:00 am and 12:00 pm.15 As with acute myocardial infarction or myocardial ischemia, physical and mental activities can also be triggers for acute aortic dissection.16
Pathophysiology of Aortic Dissection
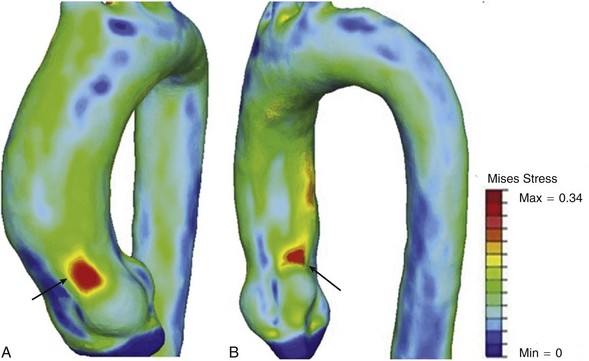
Aortic dissection is believed to occur when intraluminal wall stress exceeds aortic wall strength.17 Therefore known risk factors such as hypertension and vessel diameter increase aortic wall stress, and collagen vascular disorders contribute to a reduction of wall strength, both contributing to the pathophysiology of the disease. Acute type A dissections tend to occur at the sinotubular junction, and type B dissections occur distal to the origin of the left subclavian artery. Nathan and colleagues17 recently demonstrated, in a study using electrocardiogram-gated computed tomography angiography in 47 patients, that peaks of aortic wall stress exist in the sinotubular junction and distal to the left subclavian artery ostium, likely contributing to the development of type A and type B dissections in these locations. They also found that the wall stress proximal to the sinotubular junction exceeded that of the area distal to the left subclavian artery, thus supporting the increased prevalence of type A dissections compared with type B (Figure 38-2).
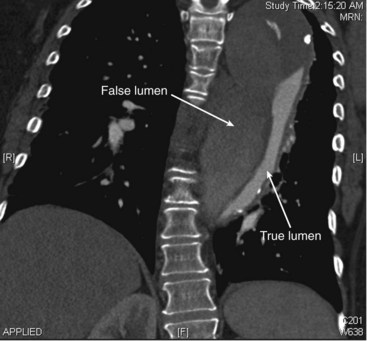
Once an intimal tear occurs, propagation of the dissection is a dynamic process that can result in both antegrade and retrograde (less common) propagation of blood flow through the false lumen. Fenestrations between the two channels connect the true and false lumen and occur downstream, usually at the origin of branch vessels. The most common location of the false lumen in type B dissections is in the posterolateral aorta (Figure 38-3, see color plate). Although significant variation occurs, the celiac trunk, the superior mesenteric artery, and the right renal artery most commonly arise from the true lumen, and the left renal artery arises from the false lumen.
The aortic wall surrounding the false lumen is significantly weaker than that of the true lumen. In the majority of patients, the false lumen has the larger diameter. A large false lumen diameter relative to the size of the true lumen has been shown to be a strong predictor for secondary dilatation in chronic dissections.18 Sueyoshi and colleagues19 followed 62 patients with chronic type B dissections and demonstrated that 83.9% of patients had one or more aortic segments increase in size during the follow-up period (mean, 49.1 months). Patients with blood flow in the false lumen had a significantly higher mean growth rate (3.3 mm/yr) than the group without blood flow (–1.4 mm/yr). The presence of blood flow in the false lumen was also the only significant risk factor for increase in the diameter in the univariate and multivariate analysis.
In a model of chronic type B dissection, Tsai and colleagues20 demonstrated that diastolic false lumen pressures were highest in the setting of smaller proximal tear size and the lack of a distal tear. Therefore, the lack of an outflow tear may lead to false lumen expansion and dissection-related morbidity and mortality. Significant atherosclerosis is relatively infrequent in patients with acute aortic dissection, and it has been hypothesized that the presence of atheromatous plaque may serve to terminate or limit the extent of the dissection. One potential theory is enhanced fusion of the aortic wall layers secondary to inflammatory aortic wall plaques.
Weakening of medial collagen and elastin fibers within the aortic wall is a pathologic process referred to as cystic medial necrosis and has been implicated in the development of aortic dissection.21 Patients with hereditary connective tissue disorders such as Marfans or Ehlers-Danlos syndromes universally demonstrate cystic medial necrosis in the aortic wall. Patients without these disorders but with aortic dissection also tend to have a higher degree of cystic medial necrosis than the general population.
Clinical Presentation
Malperfusion Syndrome
Malperfusion following aortic dissection is defined as the loss of adequate blood supply to vital organs owing to aortic branch vessel obstruction resulting in end-organ ischemia. This accompanying clinical process is a significant risk factor for increased morbidity and mortality in patients with aortic dissection.22 In a study by the IRAD investigators, 15% of all deaths following aortic dissection were related to mesenteric ischemia.23,24 Approximately one third of patients with type A dissection will also exhibit some manifestation of malperfusion syndrome.25 Malperfusion can be classified into two distinct categories based on the physiologic mechanism of obstruction: dynamic and static.
Dynamic Malperfusion
Augmented pressure within the false lumen may also contribute to significant compression of the true lumen and lead to increased dissection related-mortality.26 Tsai and colleagues demonstrated that partial thrombosis of the false lumen was associated with increased postdischarge mortality in patients with type B acute aortic dissection.24 The proposed hypothesis for this increased risk is that thrombus within the false lumen occludes distal tears, resulting in restricted outflow with a subsequent increase in false lumen pressure.18 Medical management primarily through blood pressure reduction may improve the end-organ ischemia in patients with dynamic malperfusion. Patients who demonstrate clinical signs of persistent malperfusion despite optimal medical therapy will likely require endovascular or surgical treatment.
Diagnostic Pitfalls
The diagnosis of aortic dissection may often be misleading because the common clinical signs and symptoms in these patients are often attributed to various other clinical syndromes. As many as 65% of aortic dissections are misdiagnosed upon initial presentation in the emergency department.27 Acute chest pain is commonly associated with myocardial infarction and acute coronary syndrome, and delayed or incorrect diagnosis can occur if a high index of suspicion for dissection is not present. In addition, positive electrocardiogram and serologic markers for acute myocardial infarction do not rule out associated aortic dissection. Peripheral neurologic symptoms may also be mistakenly attributed to musculoskeletal pain, neuropathy, or radiculopathy.
The following clinical scenarios should prompt immediate imaging studies to confirm the diagnosis of aortic dissection as well as urgent surgical consultation28:
• Chest pain with neurologic symptoms
• Chest pain with radiation to the back
• Chest pain with signs of arterial insufficiency
• Chest pain with associated severe back or abdominal pain
• Chest pain with widened mediastinum on chest radiograph
Diagnostic Imaging
Chest Radiography
Chest radiography may reveal the presence of a widened mediastinum and aid in the diagnosis of aortic dissection. However, 10% to 20% of patients with aortic dissection have a normal chest radiograph.29 Although chest radiographs should be obtained in all patients suspected of having this disorder, additional noninvasive imaging is usually required for confirmation of the diagnosis.
Computed Tomography
Computed tomography angiography (CTA) has become the noninvasive imaging study of choice to evaluate aortic pathology. Continued advances in CT scanning in regard to high spatial and temporal resolution have allowed comprehensive analysis of detailed elements for even the most complex dissections (Figure 38-4, see color plate).30 Several recent studies have demonstrated a sensitivity and specificity for detection of aortic dissection and aortic intramural hematoma to be near 100%.31–34 Reliable imaging of both the true and false lumens, location of the intimal flap, fenestrations and branch vessel involvement can be achieved with this diagnostic modality. Multidetector CTA with electrocardiograph-gated synchronization improves diagnosis of type A aortic dissections by decreasing motion-related artifacts in the ascending aorta and aortic valve.35 Three-dimensional reconstructions can be rendered and used for endovascular or surgical planning.
Transthoracic and Transesophageal Echocardiography
Transesophageal echocardiography (TEE) allows high resolution imaging from the aortic root to the descending thoracic aorta. The proximity of the esophagus to the aorta, as well as decreased interference from the chest wall and lung allow high-quality images of the proximal aorta to be obtained. It is superior to transthoracic echocardiography (TTE) for imaging of the intimal tear, true and false lumens, coronary artery involvement, pericardial effusion, aortic valve regurgitation, and flow within the false lumen. Several studies have demonstrated the accuracy of TEE for the diagnosis of aortic dissection with a sensitivity of 86% to 100%, specificity of 90% to 100%, and a negative predictive value of 86% to 100%.36,37 Sensitivity and specificity for TTE have ranged from 78% to 90% for ascending aortic dissections, but only 31% to 55% for descending aortic dissections. Specificity ranges from 87% to 96% for type A dissections and 60% to 83% for type B dissections.35 Although TEE is superior for definitive diagnosis of aortic dissection, TTE can be performed rapidly in the emergency room setting and may be used to reliably evaluate the location of the intimal flap in the proximal ascending aorta, pericardial effusion and tamponade, and left ventricular function. It is important to note that a negative TTE result does not rule out aortic dissection, and additional imaging is required if there is a high degree of suspicion despite negative TTE results.
Magnetic Resonance Angiography
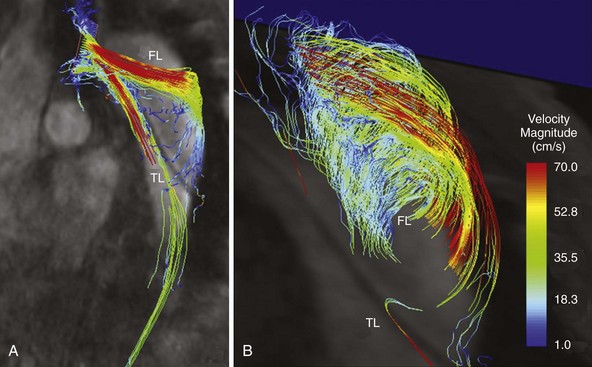
Because of long acquisition times, magnetic resonance angiography (MRA) is not as useful for immediate diagnosis of suspected acute aortic dissection. However, contrast-enhanced three-dimensional MRA has an excellent reported sensitivity and specificity (95% to 100%) for the diagnosis of this disorder.38 Similar to CTA, MRA can accurately determine the presence or absence of intimal flaps and branch vessel involvement. Information regarding false lumen perfusion can also be readily obtained and may be helpful in the evaluation of visceral ischemia and impaired branch vessel perfusion. Recently newer techniques, such as three-dimensional velocity-encoded cine magnetic resonance imaging, are being used increasingly for superior visualization of pathophysiologic hemodynamics and geometrically triggered changes in blood-flow characteristics within the dissected aortic lumens (true and false; Figure 38-5, see color plate).39 Limitations of MRA include inability to perform the study in patients with pacemakers or other metallic implants, long examination times, poor tolerance in claustrophobic patients, and the association with nephrogenic systemic fibrosis in patients with advanced chronic kidney disease.
Angiography
The routine use of angiography in patients with aortic dissection is no longer indicated because of its invasiveness and the evolution of the previously described noninvasive imaging modalities for diagnosing aortic dissection. The IRAD investigators reported an overall sensitivity of 87% with aortography for diagnosing aortic dissection.40 Sensitivities for type A and type B dissections were 87% and 89%, respectively. False negatives may also occur in the presence of intramural hematoma and a thrombosed false lumen. The main indication for aortography is for endovascular treatment of aortic branches (e.g., angioplasty, stenting), the thoracic or abdominal aorta in association with stent-grafting, or fenestration. These techniques will be discussed later in this chapter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree