CHAPTER 78 Acquired Disease of the Mitral Valve
HISTORY
The first physician to propose that operating on the diseased mitral valve could provide symptomatic relief was Daniel Samways, who in 1898 suggested in the British Medical Journal that rheumatic mitral stenosis might be amenable to surgical correction. His comments were largely ignored until 1902, when Sir Lauder Brunton published his “Preliminary Note on the Possibility of Treating Mitral Stenosis by Surgical Methods” in The Lancet, in which he proposed a closed mitral commissurotomy for treatment of rheumatic mitral stenosis based on his experimental work in cadavers. The correspondence that followed publication of his idea was highly critical, and any enthusiasm there may have been for the idea of mitral valve surgery disappeared.1 It was not until 1923 that Elliott Cutler, who had spent the previous 2 years collaborating with the cardiologist Sam Levine at the Peter Bent Brigham Hospital in Boston on this procedure, performed the operation proposed by Brunton 20 years earlier. Using a valvulotome designed for the purpose, he performed a mitral valvotomy through the left ventricular apex in a 12-year-old girl who had presented with incapacitating dyspnea and hemoptysis2; the patient’s hemoptysis resolved, and she survived another 41⁄2 years. Subsequent patients fared poorly, however, primarily because of consequent mitral regurgitation; the longest survivor died on postoperative day 7, and Cutler eventually abandoned the operation. In 1925 at the London Hospital, the English surgeon Sir Henry Souttar used a finger to perform the first closed digital commissurotomy3; his 19-year-old patient lived another 5 years. Souttar was referred no more patients despite the apparent success of the procedure, and after further unsuccessful attempts at mitral commissurotomy by Cutler, the procedure was abandoned in humans.
Work in the 1930s and 1940s focused on development of techniques of closed mitral valvotomy in experimental animal models. William Wilson at the University of Edinburgh in Scotland used a canine model to demonstrate how poorly an acute change from mitral stenosis to regurgitation was tolerated by the heart. Horace Smithy spent several years in the animal laboratory at the Medical College of South Carolina and designed a valvulotome that he used in early 1948 in six patients, of whom four survived. Smithy died on October 28, 1948, aged 34 years, of rheumatic mitral and aortic stenosis, before he was able to persuade Alfred Blalock at Baltimore to use the valvulotome on his own stenotic valves. Charles Bailey of Delaware performed more than 60 mitral valve operations in a canine model developing a commissurotomy knife as preparation for five procedures in humans, of whom four died; the last, in May 1948, survived.4 Less than a week after Bailey’s success, Dwight Harken in Boston, followed by Lord Russell Brock in England 3 months later, carried out the first successful clinical cases.
In the 1950s, several techniques of repair for mitral regurgitation without the use of cardiopulmonary bypass were attempted. In 1954, Bailey reported the correction of mitral regurgitation by use of a pericardial graft.5 He understood from work undertaken by John Gibbon of Jefferson Medical College in Philadelphia that long-term results of pericardial patch repair could be compromised by late shrinkage. Bailey believed that graft durability could be enhanced by maintaining a vascularized pedicle; with a finger in the left atrial appendage, he passed a folded tube of pedicled pericardium through the heart beneath the mitral anulus, anchoring the free end with sutures at sufficient tension to reduce mitral regurgitation. He repeated this procedure in another six patients, claiming success in six. Andrew Logan in Edinburgh developed a method of blindly suturing a sheet of pedicled pericardium to the inferior aspects of the anterior and posterior mitral orifice so that the pericardium obstructed the ventricular inflow tract during systole but not during diastole. He used this technique successfully in 11 patients in total.
Various methods of partial anular reduction were described and used by Bailey, Harken, and William Jamieson, in addition to multiple attempts to blindly place spindle- and ball-shaped baffles, which had a tendency to cause inflow obstruction. In the absence of any method of accurately visualizing the mitral valve, Robert Glover and Julio Davila in Philadelphia described the concept of anular size reduction by external circumferential suture of the mitral anulus in 1955, which they used in 27 patients by 1956, claiming symptomatic benefit in half.6 In 1956, Nichols reported his technique of anular plication at the posteromedial commissure using transatrial sutures. In 1958, he reported on 93 patients with a 27% mortality.7
The development of cardiopulmonary bypass in the early 1950s allowed surgeons to refine techniques of mitral repair and replacement. It was Lillehei in 1957 who reported the first suture mitral anuloplasty by placing heavy silk sutures in the dilated area of the mitral anulus.8 In 1959, Merendino reported the concept of posteromedial anuloplasty.9 Subsequently, Kay,10 Wooler,11 and Reed12 described other techniques of anuloplasty, but none provided good long-term results, and they were therefore abandoned. In 1966, McGoon from the Mayo Clinic reported the resection-plication of the posterior leaflet for the treatment of mitral valve insufficiency.13
In the absence of reproducible and reliable mitral repair techniques, surgeons began to focus on development of valve prostheses. Nina Braunwald and Andrew Morrow implanted a polyurethane prosthesis reinforced with Dacron in the mitral position on March 10, 1960, in a 16-year-old girl with severe mitral regurgitation at the National Institutes of Health. In addition to the leaflets, the prosthesis had extensions that could be sutured to the ventricular wall to act as neo-chordae. Despite successful outcomes in a canine model, the valve prosthesis failed to function, and their patient died within 3 days. A second patient was well 8 weeks later. Starr14 was the first to successfully implant a caged ball valve with good long-term results in 1960. During the following 2 decades, significant technological advances were made in the development of different types of reliable prostheses, such as porcine valves, tilting disc valves, and bileaflet mechanical valves.
Motivated by the limited durability of the bioprosthetic material and the high rate of thromboembolic and hemorrhagic events associated with mechanical valves, Alain Carpentier15 and Carlos Duran16 focused their interest on the development of new repair techniques. In 1968, Carpentier performed the first remodeling anuloplasty with a prosthetic ring. Additional repair techniques were developed to broaden the application of reconstructive surgery in patients with valvular disease during the following 2 decades.17,18 In the mid-1990s, research efforts further focused on the development of minimally invasive cardiac procedures. In 1996, Carpentier19 performed the first videoscopic mitral valve repair through a right mini-thoracotomy. In 1998, it was Carpentier20 and Mohr21 who performed the first mitral valve repair with robotic assistance.
ANATOMY
Valvular Tissue
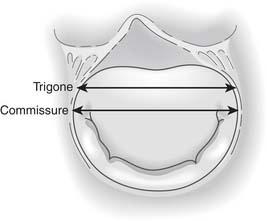
The mitral valve leaflet tissue, which inserts onto the entire circumference of the mitral anulus, consists of anterior and posterior leaflets as well as the posteromedial and anterolateral commissures (Fig. 78-1).
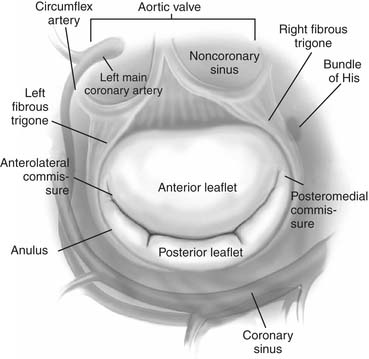
Figure 78–1 Surgical anatomy of the mitral valve with important structures surrounding the anulus.
(From Carpentier A, Adams DH, Filsoufi F. Carpentier’s Reconstructive Valve Surgery. Elsevier, 2010.)
Commissures
The posteromedial and anterolateral commissures are identified by two anatomic landmarks: the axis of the corresponding papillary muscles and the commissural chordae (Fig. 78-2). The distance between the free edge of the commissures and the anulus is approximately 8 mm. During an open commissurotomy, the surgical incision should not encroach on this distance to reduce the risk of the leaflet’s tearing as far as the anulus and resultant mitral regurgitation.
Leaflets
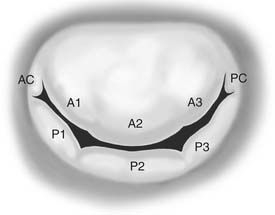
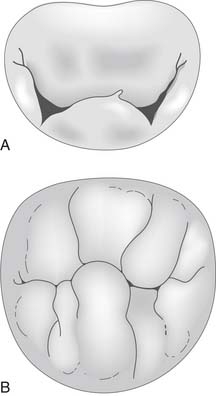
The mitral valve leaflet tissue may be further divided into eight segments (Fig. 78-3).18 The anterolateral and posteromedial commissures constitute two segments. The posterior leaflet is divided by two large indentations on its free edge into three scallops, identified as P1 (anterior scallop), P2 (middle scallop), and P3 (posterior scallop). The three corresponding segments of the anterior leaflet are A1 (anterior segment), A2 (middle segment), and A3 (posterior segment). This anatomic nomenclature facilitates precise location of valve disease (leaflet prolapse or restriction).
Mitral Anulus
The right fibrous trigone is a dense fibrous area of continuity between the mitral, tricuspid, noncoronary cusp of the aortic anuli and the membranous septum (see Fig. 78-1). The left fibrous trigone is situated at the junction of both left fibrous borders of the aortic and the mitral valves. The right and left fibrous trigones mark the far ends of the area of fibrous continuity between the aortic and mitral valves. The mitral valve leaflets, unlike the tricuspid valve leaflets, are attached to an ovoid ring or anulus of fibrous tissue extending from the right and left fibrous trigones, which constitutes the junction between the left ventricle and atrium.
The mitral anulus is thinnest at the insertion site of the posterior leaflet. This segment is not attached to any rigid structures, and it is predominantly here that anular dilatation occurs. Studies have demonstrated that moderate anular dilatation can also occur at the anterior portion of the mitral anulus between the fibrous trigones.22 The mitral anulus is closely related to the important anatomic structures highlighted in Figure 78-1.
Chordae Tendineae
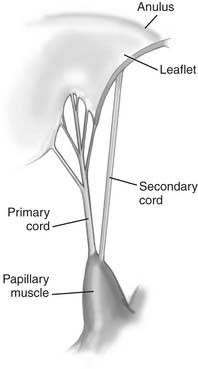
The chordae tendineae are fibrous leaflet extensions connecting the papillary muscles and the leaflets. They are classified according to the site of insertion between the free margin and the base of leaflets (Fig. 78-4). Marginal chordae (primary chordae) insert on the free margin of the leaflets and function to limit leaflet prolapse. Intermediate chordae (secondary chordae) insert on the ventricular surface of the leaflets and reduce excess tension on the valve leaflets. Basal chordae (tertiary chordae) are limited to the posterior leaflet. They are attached to the leaflet base and connect it to the mitral anulus and the surrounding myocardial tissue.
MITRAL STENOSIS
Pathophysiology
The normal mitral valve area is 4 to 6 cm2. Narrowing of this area to less than 2.5 cm2 means that a pressure gradient across the valve is required to eject blood from the left atrium into the ventricle. Mitral stenosis is considered mild when the mitral valve area is greater than 1.5 cm2, moderate when the mitral valve area is 1 to 1.5 cm2, and severe when the mitral valve area is less than 1.0 cm2 or the mean transvalvular gradient is more than 10 mm Hg. The transvalvular gradient results in elevated left atrial and pulmonary venous pressures. Pulmonary edema results when pulmonary venous pressure is greater than plasma oncotic pressure. Pulmonary artery hypertension may be caused by compensatory vasoconstriction and intimal hypertrophy of the pulmonary arterioles, together with direct transmission of the elevated pulmonary venous pressure. At rest, this is often asymptomatic, but any increase in flow across the mitral valve or reduction in the diastolic filling period results in an increase in the pressure gradient across the mitral valve. Dyspnea in these patients is therefore usually precipitated by exercise, stress, infection, pregnancy, or rapid atrial fibrillation.
Etiology
Although rheumatic valve disease remains the predominant cause of mitral stenosis in the West, with more than two thirds of patients with mitral stenosis giving a history of rheumatic fever, its prevalence has decreased in recent decades. This is in sharp contrast to the developing world, where chronic rheumatic disease is endemic and remains the most common cause of both mitral regurgitation and stenosis.23 Around one third of all patients with rheumatic heart disease have pure mitral stenosis, and the remainder have a combination of mitral valve stenosis and regurgitation.24
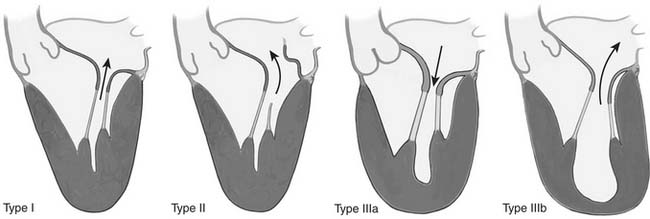
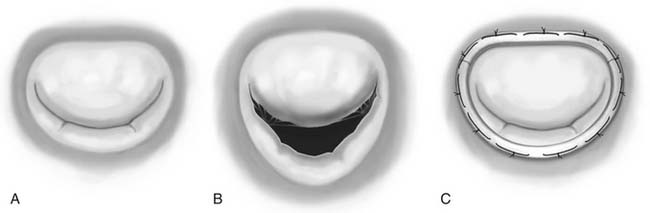
Rheumatic disease results in an insidious fibrotic process affecting all segments of the mitral apparatus. Early valvular lesions include leaflet thickening, chordal thickening, fusion and shortening, and commissural fusion. Progressive leaflet thickening and commissural fusion eventually produce a characteristic “fish mouth” single central opening, with restricted leaflet motion during systole and diastole (Carpentier type IIIa leaflet dysfunction; Fig. 78-5). Chordal thickening and fusion may result in a dense fibrotic subvalvular mass that can contribute to obstruction of forward flow. Calcification, particularly at the commissural edges and occasionally extending posteriorly into the anulus and subvalvular apparatus, is common late in the disease process and in more elderly patients. Lesions that reduce the coaptation area (leaflet retraction) or restrict leaflet mobility (chordal shortening) result in mitral regurgitation as well as in stenosis. Other diseases, such as malignant carcinoid and systemic lupus, very occasionally affect the mitral valve, causing varying degrees of mitral stenosis.
Diagnosis
The diagnostic tool of choice in the evaluation of patients with mitral stenosis is two-dimensional Doppler echocardiography.25 Echocardiography is used to evaluate the morphology and mobility of the mitral valve leaflets, commissures, and subvalvular apparatus, identifying calcification as well as determining the severity of mitral stenosis by measuring the mitral valve area, the transmitral gradient, and the pulmonary artery pressures. Rheumatic mitral valve disease is commonly associated with mixed aortic valve disease as well as tricuspid regurgitation. Transthoracic echocardiography allows assessment of other valves, evaluation of right and left ventricular function, and identification of pulmonary hypertension and intracardiac thrombus; it is used to determine the suitability of the stenotic mitral valve for percutaneous balloon valvuloplasty.
Left-sided heart catheterization is not normally necessary for diagnosis of mitral stenosis but may be useful when there is a discrepancy between noninvasive assessments of mitral stenosis; it is advisable in patients with risk factors for coronary artery disease in whom surgical correction is indicated. Right-sided heart catheterization is employed to obtain direct measurement of pulmonary artery pressures in patients in whom severe pulmonary hypertension is suspected; in combination with pulmonary vasodilators, it may be helpful in assessing reversibility of pulmonary hypertension.26
Indications for Surgery
Since the turn of the century, percutaneous mitral valvulotomy has become the first-line therapy for many patients with mitral stenosis. This procedure is indicated in symptomatic patients (New York Heart Association [NYHA] class III and IV) with isolated moderate to severe mitral stenosis (mitral valve area ≤1.5 cm2) and favorable valve morphology.27 Asymptomatic patients with moderate to severe mitral stenosis and pulmonary hypertension at rest (pulmonary artery systolic pressure >50 mm Hg) or with exercise (pulmonary artery systolic pressure >60 mm Hg or pulmonary artery wedge pressure ≥25 mm Hg) or with new-onset atrial fibrillation may also benefit from percutaneous mitral valvulotomy. Percutaneous valvulotomy is contraindicated in the setting of left atrial thrombus, moderate mitral regurgitation, or valve morphology that includes moderate or severe valvular calcification (Wilson score >10, Massachusetts General Hospital score 4) or subvalvular fusion. The Wilkins scoring system grades mitral valve morphology by use of echocardiography. Four characteristics, graded 0 to 4, are assessed: leaflet mobility, leaflet thickening, valve calcification, and involvement of the subvalvular apparatus. A total score of more than 8 is predictive of a low success with percutaneous mitral valvuloplasty.28 Percutaneous mitral valvulotomy is associated with recurrent mitral stenosis, especially in patients undergoing repeated procedures, and with iatrogenic mitral regurgitation in patients with high valve scores.29
In a low-risk surgical patient with moderate to severe mitral stenosis (mitral valve area ≤1.5 cm2) who is not appropriate for or who has failed balloon valvulotomy, NYHA class III or class IV symptoms are an American College of Cardiology/American Heart Association (ACC/AHA) class I indication for mitral valve surgery (Fig. 78-6).27,30 There is also a subset of less-symptomatic patients with severe mitral stenosis and severe pulmonary hypertension (pulmonary artery systolic pressures >60 mm Hg) with morphology unfavorable for percutaneous balloon valvulotomy who may benefit from surgery. Mitral valve surgery is recommended in this subgroup of patients to prevent right ventricular failure. Currently, the ACC/AHA guidelines do not recommend surgery in asymptomatic patients.
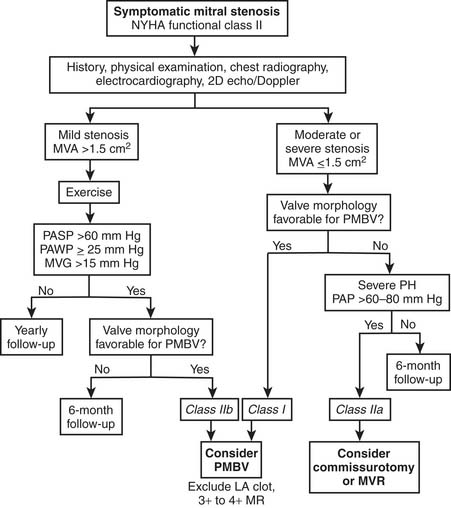
(From Bonow RO, Carabello B, de Leon AC, et al. ACC/AHA guidelines for the management of patients with valvular heart disease. Executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [Committee on Management of Patients With Valvular Heart Disease27].)
In patients with mild asymptomatic mitral stenosis (valve area >1.5 cm2 and mean gradient <5 mm Hg), no further evaluation is required after the initial workup. These patients usually remain stable for years and should be treated medically with follow-up every 6 months if they are not candidates for percutaneous balloon mitral valvotomy.
MITRAL REGURGITATION
Pathophysiology
Mitral regurgitation, the retrograde ejection of blood from the left ventricle into the left atrium during systole, may progress through one or more of three stages: acute mitral regurgitation, chronic compensated mitral regurgitation, and chronic decompensated mitral regurgitation.31 Mitral regurgitation results in volume overload of the left ventricle at the end of diastole (i.e., increased preload) as well as in a reduction of afterload due to the regurgitant pathway back into the left atrium. As a result of the increased preload and reduced afterload, a larger volume of blood is ejected from the left ventricle. However, because a proportion of ejected blood enters the left atrium rather than the aorta, the forward stroke volume and consequently the cardiac output decrease. The increased blood volume in the left atrium raises the pressure from a normal left atrial pressure of about 10 mm Hg to up to 25 mm Hg. Increased preload gradually leads to ventricular remodeling through eccentric hypertrophy and dilatation, increasing the total stroke volume and the forward stroke volume, which may return to almost normal levels. This is chronic compensated mitral regurgitation.
Anular dilatation occurs as a result of ventricular dilatation. The normal ratio between the anteroposterior and transverse diameters of the mitral anulus is 3:4 in systole. This ratio inverts in patients with chronic mitral regurgitation, causing poor leaflet coaptation and regurgitation even in the absence of leaflet prolapse (Fig. 78-7). This progressive defect affects the anterior anulus to a lesser extent than the posterior anulus.
Carpentier’s Functional Classification
Accurate description of valve disease is facilitated by the use of the pathophysiologic triad first described by Carpentier (Table 78-1). This triad is composed of etiology (cause of the disease), valve lesions (structural changes resulting from the disease), and leaflet dysfunction (changes in leaflet motion resulting from the structural lesion; see Fig. 78-5). These distinctions are relevant because long-term prognosis depends on etiology, treatment strategy depends on the valve dysfunction, and surgical techniques are dictated by the valve lesions.32
Table 78–1 Pathophysiologic Triad
| Disfunction | Lesions | Etiology |
|---|---|---|
| Type I | ||
| Normal leaflet motion | ||
| Type II | ||
| Increased leaflet motion (leaflet prolapse) | Elongation or rupture of chordae | |
| Elongation or rupture of papillary muscle | Ischemic cardiomyopathy | |
| Type IIIa | ||
| Restricted leaflet motion (systole and diastole) | ||
| Type IIIb | ||
| Restricted leaflet motion (systole) | ||
Carpentier’s functional classification is used to describe the mechanism of mitral regurgitation.17 This classification is based on the opening and closing motions of the mitral leaflets. Patients with type I dysfunction have normal leaflet motion. Mitral regurgitation in these patients is due to anular dilatation or leaflet perforation. In type II dysfunction, leaflet motion is increased, with the free edge of the leaflet overriding the plane of the anulus during systole (leaflet prolapse). The most common lesions responsible for type II dysfunction are chordal elongation and rupture, followed by papillary muscle elongation and rupture. In type IIIa dysfunction, leaflet motion is restricted during both diastole and systole. The most common lesions are leaflet thickening or retraction; chordal thickening, shortening, or fusion; and commissural fusion. Type IIIa regurgitation is most often associated with some degree of mitral stenosis. The mechanism of mitral regurgitation in type IIIb dysfunction is restricted leaflet motion during systole due to left ventricular enlargement with posterior displacement of the apex of the papillary muscle.
Etiology
Degenerative Disease
Degenerative mitral valve disease is the most common cause of mitral regurgitation in Western countries. Degenerative disease is defined as a spectrum of conditions in which infiltrative or dysplastic tissue changes cause elongation or rupture of the mitral valve chordae, resulting in leaflet prolapse or anular dilatation (Fig. 78-8).33 The main mechanism of mitral insufficiency is type II dysfunction (leaflet prolapse).17,34–37 However, type I dysfunction with isolated anular dilatation has also been reported. Causes of degenerative mitral valve disease include fibroelastic deficiency, Barlow’s disease, and Marfan syndrome.38,39 In some cases, the exact cause remains undetermined.
Fibroelastic deficiency is most common in elderly patients with a relatively short history of mitral regurgitation. Fibroelastic deficiency occupies one end of the spectrum of degenerative disease (see Fig. 78-8) and is characterized by insufficient tissue in a normal-sized valve; leaflets are thinned, almost transparent, and chordae are flimsy and elongated. Regurgitation is most often caused by rupture of a single cord associated with a single thickened, prolapsing segment, usually P2, resulting in type II leaflet dysfunction. Tissue resection must be approached with caution, as the surgeon runs the risk of leaving insufficient leaflet tissue to complete a successful repair.38
Barlow’s disease appears early in life, and patients typically have a long history of a systolic murmur. Barlow’s disease, which occupies the opposite end of the spectrum of degenerative mitral regurgitation to fibroelastic deficiency, is characterized by excess tissue in a very dilated valve (see Fig. 78-8). Leaflet tissue is thickened, and there is obvious redundancy in multiple segments, with thick, elongated, meshlike chordae and occasionally papillary muscle elongation.40 Regurgitation is due to the multiple areas of prolapse (type II leaflet dysfunction). The anulus is dilated and may be calcified. Aggressive tissue resection and chordal reconstruction are mainstays of repair in these complex and challenging valves. On histologic examination, there is extensive myxoid degeneration with destruction of the normal three-layer leaflet tissue architecture.38 Forme fruste valves share morphologic features of Barlow’s disease and fibroelastic deficiency, with multiple areas of leaflet thickening and prolapse within a small valve.
Marfan syndrome with mitral regurgitation is characterized by excess leaflet tissue, which may be thickened (without myxoid degeneration), and a dilated anulus that is rarely calcified.41 It is commonly associated with aortic regurgitation due to anular dilatation.
Rheumatic Heart Disease
The mechanism of mitral regurgitation is usually type IIIa dysfunction.42–44 Leaflet thickening and retraction and chordal shortening and fusion are common lesions, which all reduce leaflet motion during diastole and systole, leading to mitral regurgitation and mitral stenosis. Commissural fusion and leaflet thickening further reduce leaflet mobility during diastole, contributing to mitral stenosis. On occasion, anterior leaflet chordal elongation causes type II dysfunction; anterior leaflet prolapse and posterior leaflet restriction (type II anterior, type III posterior) is one of the most common mechanisms of mitral regurgitation in young rheumatic patients.42
Endocarditis
Mitral valve endocarditis classically occurs in patients with a structurally abnormal valve due to underlying degenerative or rheumatic valve disease, but several organisms (e.g., Staphylococcus aureus) are capable of infecting completely normal valves. The principal organisms involved in native endocarditis are Streptococcus viridans, Streptococcus bovis, and S. aureus.45 Native mitral valve endocarditis may result in vegetations, chordal rupture, leaflet abscess and perforation, and anular abscess.46–50 Aortic valve endocarditis may also cause native mitral valve endocarditis by direct extension of the infective process51; an aortic anular abscess may extend to the mitral anulus and onto the anterior leaflet of the mitral valve. In aortic endocarditis, the diastolic jet of aortic regurgitation occasionally results in secondary mitral endocarditis, with vegetations or leaflet perforation known as kissing lesions forming on the ventricular surface of the anterior leaflet.
Trauma
Percutaneous mitral valvotomy may be complicated by leaflet injury and chordal rupture, causing traumatic mitral regurgitation.52,53 Blunt or penetrating chest trauma is a rare cause of mitral regurgitation that typically results from chordal rupture.
Ischemic Cardiomyopathy
Ischemic mitral regurgitation is discussed in detail in Chapter 91. Ischemic cardiomyopathy may result in mitral regurgitation by several mechanisms. Carpentier’s type IIIb dysfunction is the most common form of ischemic mitral regurgitation, resulting from several anatomic and physiologic changes: left ventricular remodeling after myocardial infarction, left ventricular wall motion abnormalities, papillary muscle infarction, and mitral anulus dilatation.54–59 After myocardial infarction, left ventricular remodeling converts the ventricular shape from ellipsoidal to spherical. This process leads to papillary muscle displacement, which causes a restriction of posterior leaflet motion during systole (tethering effect), reducing the leaflet coaptation surface area and leading to mitral regurgitation.60,61 In some patients, further ventricular remodeling and dilatation cause anular dilatation, which may worsen mitral regurgitation. Type I dysfunction with anular dilatation occurs in basal myocardial infarction. Type II dysfunction results from papillary muscle rupture, which usually involves the posteromedial papillary muscle.62–64 In some instances, the necrotic papillary muscle does not rupture and becomes fibrotic. The fibrotic papillary muscle is usually elongated and causes leaflet prolapse, particularly in the commissural area.
Dilated Cardiomyopathy
Dilated cardiomyopathy is one of the most common causes of end-stage heart failure. The etiology of this disease is unknown in many cases; known causes include chronic atrial fibrillation, viral infection, excessive alcohol consumption, and immunologic abnormalities. The natural history of dilated cardiomyopathy is often complicated by secondary or so-called functional mitral regurgitation, which has a negative impact on survival.65,66 The mechanism of mitral regurgitation is type IIIb dysfunction.60,67–70
Calcifying Disease of the Anulus
Calcification is due to a degenerative process involving the base of the leaflets. Calcification is localized at the posterior part of the anulus and may extend toward the base of the left ventricle. This process is often seen in elderly patients.39,71–73 The mechanisms of mitral regurgitation are the loss of anular contraction and the restricted motion of the posterior leaflet.
Endomyocardial Fibrosis
Endomyocardial fibrosis is characterized by a progressive fibrosis of the ventricular endocardium resulting in a restrictive cardiomyopathy. The exact etiology of this disease is unknown. Mitral insufficiency is related to the involvement of the posterior leaflet and the subvalvular apparatus by the fibrotic process.74,75 Posterior leaflet restriction in the opening position is the most frequently encountered valve dysfunction (type IIIa).
Diagnosis
History and examination alone are of limited use to even experienced clinicians in the diagnosis of mitral regurgitation,76 but the presence of symptoms or evidence of atrial fibrillation, pulmonary hypertension, or embolic disease is of direct relevance to the decision of whether and when to refer for surgery. Chronic mitral regurgitation may be asymptomatic for many years. The most common presenting symptoms are fatigue, decreased exercise capacity, dyspnea, and palpitations. The history of presentation is helpful in distinguishing not only between ischemic and degenerative mitral regurgitation but also between the subtypes of degenerative mitral regurgitation; patients with Barlow’s disease are typically younger, with a long history of a murmur, compared with those with fibroelastic deficiency, who are usually older, with a relatively short history of mitral regurgitation.
Two-dimensional echocardiography is essential to determine the mechanism and the severity of mitral regurgitation,77 which may be described most precisely by segmental nomenclature and Carpentier’s functional classification. Semiquantitative assessment of regurgitant flow by maximal jet length, area, and ratio of jet to left atrial area has been used to grade the severity of mitral regurgitation. The degree of mitral regurgitation is determined by assessing jet geometry and area in multiple views. The severity of mitral regurgitation is graded on a scale of 1+ to 4+ (1+, trace; 2+, mild; 3+, moderate; 4+, severe mitral regurgitation with flow reversal in the pulmonary veins). The direction of the jet is a good indicator of the mechanism of mitral regurgitation. In type II dysfunction (prolapse), the direction of the jet is opposite to the prolapsing leaflet. With restricted leaflet motion, the direction of the jet is either central or toward the restricted leaflet.
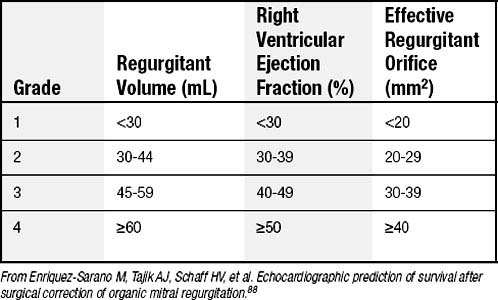
More recently, quantitative Doppler methods have allowed quantitative grading of mitral regurgitation.55,78 This quantitative grading is based on the calculation of regurgitant volume (the difference between the mitral and aortic stroke volumes) and effective regurgitant orifice (ratio of regurgitant volume to regurgitant time-velocity integral). Table 78-2 shows the relationship between the semiquantitative and quantitative grading of mitral regurgitation in degenerative mitral disease. Quantitative Doppler grading of mitral regurgitation seeks to standardize assessment of mitral regurgitation. Calculation of regurgitant volume by proximal isoelectric surface area, vena contracta width, and effective regurgitant orifice is a useful adjunct in a detailed assessment of mitral regurgitation. An effective regurgitant orifice of more than 40 mm2 is accepted as the cutoff for severe mitral regurgitation in the setting of degenerative disease. Reproducible and reliable echocardiographic assessment of the severity of mitral regurgitation remains challenging, however, and there is no “gold standard” measurement with which to judge relative accuracy.79
Table 78–2 Selected Ranges for Grading Severity of Mitral Regurgitation in Patients with Degenerative Disease
Transesophageal echocardiography should be considered in selected patients including those with complex degenerative disease and those with native valve endocarditis.80 Transesophageal echocardiography has a significantly higher sensitivity and specificity for detection of perivalvular infection and vegetations and is routinely used in intraoperative assessment of the mitral valve. Mitral regurgitation is a dynamic condition highly dependent on preload and afterload; under conditions of reduced preload and afterload, such as occurs under general anesthesia during intraoperative transesophageal echocardiography, mitral regurgitation is frequently downgraded,54 and stress echocardiography may be the only means of detecting severe mitral regurgitation in some patients. When mitral regurgitation appears insignificant on pre-bypass intraoperative echocardiography, it may be helpful to administer pressors and volume to recreate physiologic conditions in the awake patient and to review the preoperative transthoracic echocardiogram.
The indications for cardiac catheterization are similar to those described for mitral stenosis, although right-sided heart catheterization may be particularly useful in quantifying pulmonary artery and systolic and wedge pressures. Three-dimensional echocardiography and cardiac magnetic resonance imaging currently play an increasing role in the determination of the mechanism and severity of mitral regurgitation.81,82 Three-dimensional echocardiography has already yielded insights into mechanisms of mitral regurgitation, such as the differences in leaflet tenting, tethering, and stresses in anterior infarction, in which tenting is more symmetrical, compared with posterior infarction, in which these changes tend to occur in the P3 region.83 Three-dimensional echocardiography provides a surgical view of the mitral valve in which even subtle areas of prolapse or restriction may be immediately identified.
Indications for Surgery
Acute severe mitral regurgitation is an indication for urgent surgery. The indications for surgery in chronic severe mitral regurgitation have evolved during the last decade, reflecting incremental improvements in safety and efficacy of mitral valve repair as well as better understanding of long-term outcomes in medically treated patients. Factors determining the timing of surgery for isolated severe mitral regurgitation in the current ACC/AHA guidelines include symptoms, left ventricular ejection fraction, left ventricular end-systolic dimension, atrial fibrillation, and pulmonary hypertension (Fig. 78-9).84
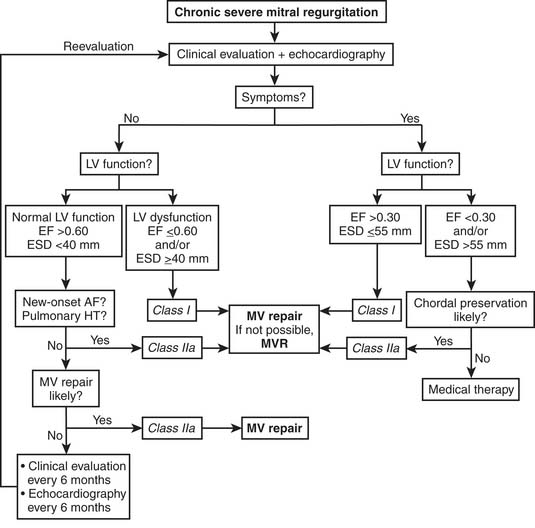
(From Bonow RO, Carabello B, de Leon AC, et al. ACC/AHA guidelines for the management of patients with valvular heart disease. Executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [Committee on Management of Patients With Valvular Heart Disease].27)
Symptoms
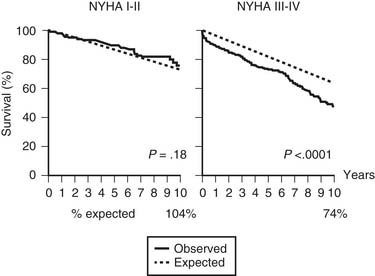
Symptomatic congestive heart failure, even if it is only transient, is an indication for urgent intervention in the context of chronic severe mitral regurgitation. The annual mortality of medically managed patients in NYHA functional class III or class IV was shown in one study to be more than 30%, even if symptoms were only transient (Fig. 78-10).85 An increasing number of clinicians advocate operating earlier in less-symptomatic patients because NYHA functional class has been shown to be an independent predictor of postoperative mortality and left ventricular dysfunction.86 In ostensibly asymptomatic patients, stress testing may occasionally be helpful.84 Evidence of deteriorating left ventricular function, new-onset atrial fibrillation, and raised pulmonary artery pressures in asymptomatic and NYHA class II patients with chronic severe mitral regurgitation are ACC/AHA indications for surgery (see later). Asymptomatic patients with severe mitral regurgitation but preserved left ventricular function should not, however, undergo surgery if there is a less than 90% likelihood of successful repair in view of the poorer outcomes associated with mitral replacement.84
Left Ventricular Ejection Fraction
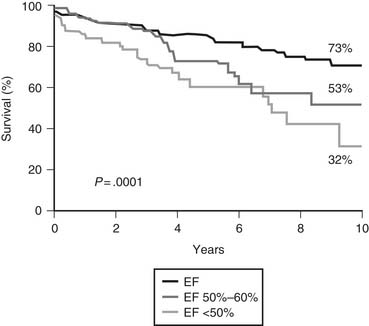
Preoperative left ventricular ejection fraction is one of the strongest predictors of outcome after surgery for chronic mitral regurgitation.86,87 Preoperative ejection fraction has been shown to be a significant predictor of overall survival (Fig. 78-11),87,88 postoperative ventricular function,86,87 and functional status.89 In patients with severe left ventricular dysfunction, long-term results after repair are significantly better compared with medical treatment. Even though the operative risk is higher in this group of patients, poor left ventricular function is therefore no longer considered a contraindication to mitral valve repair. If, however, non–chordal-sparing valve replacement is the likely surgical technique, ACC/AHA guidelines recommend medical therapy for patients with very poor left ventricular function. In asymptomatic patients with severe mitral regurgitation, the ACC/AHA guidelines recommend surgery for patients with left ventricular ejection fractions below 60% (class I) and suggest that patients with ejection fractions above 60% should be monitored with echocardiography at regular intervals if they have no other indications for surgery. Mitral valve repair should subsequently be undertaken if left ventricular ejection fraction deteriorates.84
Left Ventricular End-Systolic Diameter
Left ventricular end-systolic diameter and left ventricular end-systolic volume are relatively independent of hemodynamic status and are readily reproducible measures of left ventricular function that can be obtained noninvasively. Patients with a left ventricular end-systolic diameter of 45 mm are highly likely to have abnormal postoperative left ventricular function.90 Similarly, a left ventricular end-systolic volume index above 50 mL/m2 has been shown to be predictive of persistent postoperative left ventricular dilatation as well as decreased survival.87 In asymptomatic patients with severe mitral regurgitation and left ventricular end-systolic diameter of 40 mm or more, ACC/AHA guidelines therefore recommend mitral repair (class I).84
Atrial Fibrillation
Atrial fibrillation is associated with significant excess morbidity and mortality and occurs in more than a quarter of medically treated patients.85 Persistent or permanent atrial fibrillation, which is less likely than paroxysmal atrial fibrillation to be successfully converted to sinus rhythm by surgery, occurs almost exclusively in patients with a left atrial diameter of more than 45 mm.91 The ACC/AHA guidelines therefore advocate surgery in asymptomatic patients with severe mitral regurgitation and new-onset atrial fibrillation (see Fig. 78-9). Surgical ablation should be a standard adjunct to mitral repair in patients with a history of atrial fibrillation because it has been shown to provide a freedom from atrial fibrillation at 1 year of almost 70%.92–94
Raised Pulmonary Artery Pressures
Preoperative pulmonary artery hypertension is a risk factor for early postoperative mortality, poorer functional status, and reduced long-term survival95 as well as poorer postoperative indices of left ventricular function.87,96 ACC/AHA guidelines recommend surgery in asymptomatic patients with severe mitral regurgitation and pulmonary artery pressures above 50 mm Hg at rest or above 60 mm Hg during exercise, irrespective of left ventricular function.
Endocarditis
Surgical therapy has dramatically improved both morbidity and mortality in the setting of endocarditis.49,50 The timing of surgery is also a critical issue in the appropriate management of this condition. Patients should be taken to the operating room regardless of the duration of antimicrobial therapy when there is an indication for surgery. However, surgery should be delayed when recent neurologic injuries are present (at least 2 weeks for ischemic injury and 4 weeks for hemorrhagic lesions).97 Current ACC/AHA class I indications for surgery in native valve endocarditis include (1) any acute valve lesion resulting in heart failure; (2) aortic or mitral insufficiency with hemodynamic evidence of elevated left ventricular end-diastolic or left atrial pressures or moderate pulmonary hypertension; (3) structural complications, such as anular or aortic abscess, or other destructive penetrating lesions, including fistulas and kissing lesions; and (4) fungal or other highly resistant organisms. Native valve endocarditis complicated by recurrent emboli and persistent vegetations despite appropriate antibiotic therapy is a class IIa indication for surgery, and the presence of mobile vegetations in excess of 10 mm with or without emboli is classed by the ACC/AHA guidelines as a class IIb indication for surgery.
SURGERY
Perioperative Management
Standard techniques of monitoring are used in patients undergoing mitral valve surgery. A pulmonary artery catheter should be placed in cases of complex mitral valve reconstructive surgery, multivalve surgery, and combined mitral and coronary artery bypass grafting surgery and in patients with increased operative risk (such as patients with left ventricular dysfunction, with pulmonary hypertension, or undergoing reoperative surgery). Carbon dioxide insufflation is routinely used to reduce the risk of air embolism after removal of the aortic crossclamp. Transesophageal echocardiography should be performed before initiating cardiopulmonary bypass in all patients to determine the mechanism and severity of mitral regurgitation (Fig. 78-12) and to assess left ventricular function, quality of repair, and de-airing of the cardiac cavities at the completion of the procedure.98 External defibrillator pads are placed in patients undergoing reoperative surgery or minimally invasive procedures for defibrillation. A double-lumen endotracheal tube is inserted in right thoracotomy approaches. An epiaortic scan of the ascending aorta is recommended in elderly patients with associated atherosclerotic risk factors and in those undergoing combined mitral valve and coronary artery bypass grafting surgery before arterial cannulation.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree