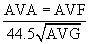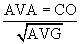CHAPTER 77 Acquired Aortic Valve Disease
FUNCTIONAL ANATOMY OF THE AORTIC VALVE
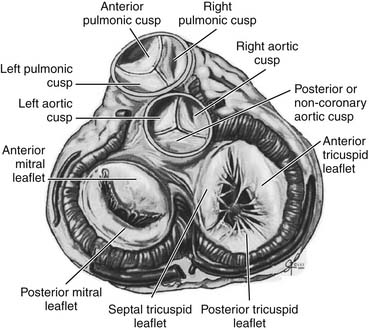
Surgically important anatomic relations of the aortic valve include the base of the anterior mitral valve leaflet, the membranous septum, and the atrioventricular conduction system of the heart (Fig. 77-1). Knowledge of these relations is essential to safe aortic valve surgery, as injury may occur during valve débridement, in suture placement, or secondary to distortion of the structures by an aortic prosthesis.
AORTIC STENOSIS
The most common indication for operation on the aortic valve is aortic stenosis, and the most common cause of aortic stenosis is degenerative valve calcification.1 Although small amounts of collagen disruption and calcific deposits on aortic valves are common in people without clinically evident aortic valve disease, significant aortic valve calcification is rare before the age of 30 years in patients with tricuspid aortic valves. Accelerated calcification may occur in patients with bicuspid or unicuspid valves. Calcific aortic stenosis is histopathologically similar to atherosclerotic coronary disease and shares similar risk factors.2 This has prompted research into the potential for lipid-lowering therapy to alter the progression of calcific aortic stenosis.
Congenital alteration in the number and orientation of the aortic valve leaflets may result in bicuspid, unicuspid, or quadricuspid morphology. Of these, bicuspid valves are most common and are present in approximately 2% of the population (Fig. 77-2).
The calcification present in stenotic aortic valves may be limited to the leaflets or extend into the anulus, interventricular septum, or anterior leaflet of the mitral valve. Even mature lamellar bone formation may occur in very calcified aortic valves. Removal of invasive calcium requires care to avoid injury to surrounding structures; when injury occurs, reconstruction of the anulus with autologous pericardium or other material may be necessary (Fig. 77-3).
Pathophysiology
The normal aortic valve has a negligible gradient during ventricular systole. In aortic stenosis, left ventricular afterload is increased, increasing left ventricular wall stress. Over time, compensatory concentric ventricular hypertrophy develops, and ejection fraction may be preserved even in the face of transvalvular gradients above 100 mm Hg. The pressure differential across the valve may be measured directly during left-sided heart catheterization or more commonly by echocardiography. Aortic valve area (AVA) may be calculated according to the Gorlin formula.3
Compensatory Changes in Aortic Stenosis
The compensatory process that results in left ventricular hypertrophy in the face of increased left ventricular afterload is poorly understood; however, the result is an increase in wall thickness, resulting in a significant increase in overall left ventricular mass from 150 g/m2 to more than 300 g/m2.4,5 In patients with severe aortic stenosis without other concomitant cardiac disease, left ventricular hypertrophy results in relative preservation of ventricular volume (concentric hypertrophy).6 In chronic severe aortic stenosis, hypertrophy may be insufficient to compensate for increasing wall stress, leading to progressive dilation and thinning of the ventricle with resultant decrease in ejection fraction and congestive heart failure.7
Diagnosis and Grading
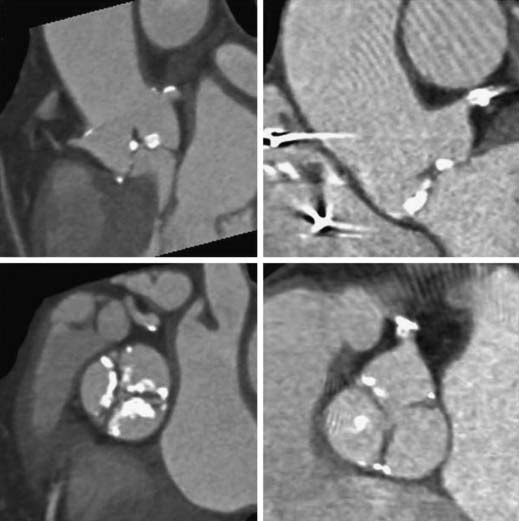
Noninvasive measurement of aortic valve gradient extrapolated from aortic jet velocity on two-dimensional echocardiography has been shown to be the most reproducible and accurate method of grading aortic stenosis (Table 77-1), such that catheter measurement of aortic valve gradients is uncommon except in select cases (see low-gradient, low-flow aortic stenosis later).8 Reports have suggested that gated cardiac computed tomography (CT) may provide more precise measurements of aortic valve morphology and area than echocardiography does.9 Evaluation of aortic stenosis by gated CT may be of particular value in patients being considered for transcatheter aortic valve implantation because it provides data on the anatomic relationship between leaflet calcification and the coronary ostia (Fig. 77-4). Cardiac CT, cardiac magnetic resonance imaging, and three-dimensional echocardiography all have the potential to provide direct (anatomic) measurement of the aortic valve area. However, they may underestimate the functional severity of aortic stenosis, which depends on the dynamic interaction between the ejecting ventricle, outflow tract, and aortic valve orifice.
Table 77–1 Grading of Aortic Stenosis
| Grade | Aortic Valve Area (cm2) |
|---|---|
| Mild | >1.5 |
| Moderate | 1-1.5 |
| Severe | <1 |
Natural History
Much has been learned about the natural history of aortic stenosis since the landmark study published by Ross and Braunwald in 1968.10 The classic symptom triad of angina, syncope, and dyspnea has since served as a hallmark for the evaluation of patients with aortic stenosis. These investigators reported a survival of 3 years for angina and syncope, 2 years with dyspnea, and 1.5 years with heart failure. These dismal survival statistics in untreated symptomatic aortic stenosis, corroborated by a number of subsequent studies,11–13 have since driven recommendations for aggressive operative therapy in patients with symptomatic severe aortic stenosis. The appropriate treatment of asymptomatic patients is more controversial, however. Estimates of the rate of sudden death in asymptomatic aortic stenosis range from 1% per year to 3% to 5% per year.14 In addition, one third of asymptomatic patients with severe aortic stenosis will become symptomatic within 2 years. Two thirds of patients will proceed to aortic valve replacement or cardiac death within 5 years of diagnosis.
Medical Treatment
Given the pathologic link between aortic stenosis and coronary disease, there has been enthusiasm for use of lipid-lowering drugs to slow the progression of leaflet disease. Despite high expectations, results have been mixed. The SEAS study, a randomized controlled trial of 1873 patients with mild to moderate asymptomatic aortic stenosis receiving simvastatin and ezetimibe or placebo, failed to show any reduction in aortic stenosis–related events in the lipid-lowering arm.2 Efforts to find a medical treatment that is effective in reducing severity or progression of aortic stenosis are ongoing. At present, medical treatment of aortic stenosis is aimed at hemodynamic stabilization in asymptomatic patients and control of comorbid conditions.
Hypertension control may help alleviate left ventricular hypertrophy. However, overmedication raises the risk of decompensation in patients who are dependent on already reduced diastolic pressure for coronary perfusion in a hypertensive ventricle. Angiotensin-converting enzyme inhibitors have been shown to provide short-term benefit in hypertensive patients with aortic stenosis.15 Atrial fibrillation may have a significant adverse impact in patients with aortic stenosis who are particularly dependent on atrial contraction for diastolic filling; therefore, cardioversion and rhythm control are important considerations both before and after aortic valve replacement. Endocarditis prophylaxis is indicated in all patients with aortic stenosis.
Timing of Intervention for Aortic Stenosis
Published guidelines support aortic valve replacement in symptomatic patients with severe aortic stenosis.16 Patients without clear symptoms or with symptoms but equivocal echocardiographic findings present a clinical challenge. In these patients, the surgeon must weigh the individual risks of aortic valve replacement with watchful waiting, which may include a potential for sudden cardiac death as well as ongoing left ventricular remodeling. Left ventricular hypertrophy as measured on routine electrocardiography is an independent predictor of symptom development in severe aortic stenosis, albeit with low sensitivity.17 Development of symptoms during exercise stress testing is a predictor of symptom development within 12 months.18 Onset of symptoms in a patient with severe aortic stenosis who has been previously asymptomatic portends a poor prognosis; however, patients often subconsciously reduce their activity levels and may therefore fail to report “symptoms.” Exercise testing may be particularly valuable in these patients.19
Lung and colleagues20 estimate that at least one third of patients with symptomatic, severe isolated aortic stenosis do not undergo surgical repair. These data were confirmed in a study from Loma Linda.1 Reasons reported for failure of patients to undergo aortic valve replacement were patient refusal and failure to refer for surgery secondary to perceived operative risk. Thus, timely surgery for asymptomatic patients could conceivably reduce the likelihood of being deemed a nonsurgical candidate when symptomatic.
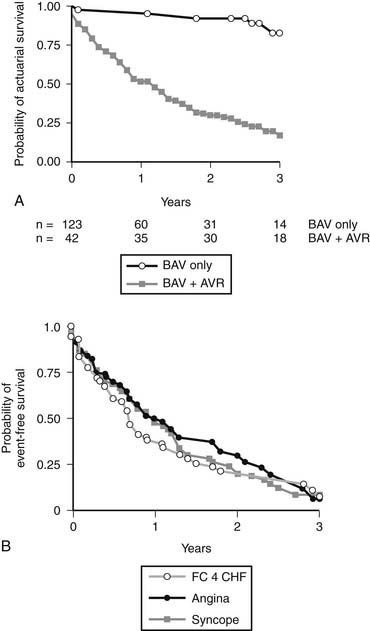
Percutaneous balloon aortic valvuloplasty has a very limited role to palliate severe aortic stenosis in patients otherwise too ill to undergo aortic valve replacement.21 Patients treated with balloon aortic valvuloplasty alone have a markedly shorter survival than patients treated with valvuloplasty followed by surgery (Fig. 77-5). Most benefits of the procedure are lost by 6 months after intervention.22 The primary limitations of balloon aortic valvuloplasty are the creation of aortic insufficiency, which may be very poorly tolerated by a hypertrophic ventricle, and restenosis. In the future, these limitations may be addressed with the use of transcatheter valved stents (see later).
Low-Gradient, Low-Flow Aortic Stenosis
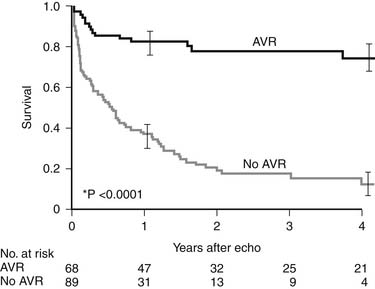
In this subset of patients with anatomic aortic stenosis who do not demonstrate increased gradients at rest (<40 mm Hg), operative mortality may be as high as 18%, with a 3-year survival of only 57%.23 Essential to the workup of these patients is determination of whether the cause of ventricular dysfunction is secondary to ischemic scar, cardiomyopathy, or excessive afterload. Low-dose dobutamine stress echocardiography serves to diagnose those patients with adequate contractile reserve, defined as an increase in stroke volume of 20% or more from baseline. Absence of adequate contractile reserve is predictive of perioperative mortality. The TOPAS multicenter study of low-flow, low-gradient aortic stenosis suggests that patients with this syndrome may benefit from aortic valve replacement with an aortic valve area as high as 1.2 cm2, suggesting that even moderate aortic stenosis may be poorly tolerated in the setting of lower left ventricular contractile reserve. Most significant risk factors for poor outcome were impaired functional capacity as measured by Duke Activity Status Index or 6-minute walk test distance, more severe stenosis, and reduced peak stress left ventricular ejection fraction. However, both functional status and left ventricular ejection fraction improved in patients surviving aortic valve replacement.24 Properly selected patients fare better with aortic valve replacement than with medical therapy (Fig. 77-6).
AORTIC REGURGITATION
Etiology
Congenital bicuspid valve disease may produce insufficiency by fibrosis and calcification in a manner similar to a degenerated calcified tricuspid valve. In addition, distortion and stretching of the leaflets may result in mal-coaptation, in which one leaflet overrides another, leading to an eccentrically directed regurgitant jet.25
Less common causes of regurgitation are injury to the valve from blunt or penetrating chest trauma, iatrogenic injury to the leaflets during catheter procedures, and suture injuries during mitral valve surgery.26
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree