Access Management Including Closure Devices
Robert J. Applegate MD, FACC, FAHA, FSCAI
Vascular access is required for all percutaneous endovascular procedures, and management of the access site is necessary at the completion of these procedures. Although access itself is simply a means to an end, unfortunate and potentially life-threatening complications can arise at the access site independently of the outcomes of the actual endovascular procedure. Accordingly, it is crucial to the success of the endovascular procedure that optimal technique and best practices be employed when gaining vascular access, and when obtaining hemostasis of the access site.
Because of its relative ease of access and size, accommodating most diagnostic and interventional catheters and devices, the femoral artery access site has been preferred traditionally over brachial and radial access (Table 29-1). Additionally, the location of the common femoral artery over the femoral head of the hip allows hemostasis to be achieved by manual compression using the femoral head as an anvil against which the femoral artery can be compressed. Although clamps and external compressive devices were developed to improve the effectiveness and safety of manual compression, manual compression today is performed the same way as it was when percutaneous catheterization was developed in the 1950s. In the early 1990s, use of large bore sheaths for atherectomy and aggressive anticoagulation for the first generation of stents severely challenged the safety and effectiveness of traditional manual compression of femoral artery access sites. In response to the larger bore arterial sheaths as well as intense anticoagulation regimens, vascular closure devices (VCDs) were developed in the hopes of achieving effective immediate hemostasis in the catheterization laboratory, accelerating the time to ambulation and reducing vascular complications following hemostasis.
ACCESS CONSIDERATIONS AND TECHNIQUES
Femoral Artery
The original percutaneous method of obtaining vascular access was pioneered by Seldinger in the 1950s (1). The original method involved performing a posterior wall stick with a needle and stilette, removal of the stilette, and withdrawal of the needle until blood exited the hub of the needle, followed by introduction of a wire into the vascular space. Over time, the technique for femoral artery access has been modified with emphasis on obtaining an anterior wall stick, to help minimize potential complications arising from posterior wall access and/or injury. By contrast, radial artery access is most commonly achieved using a posterior wall approach, although smaller-caliber needles and wires are used compared with brachial or femoral artery access. Traditionally, after local anesthesia with 1% lidocaine, an 18-gauge needle has been used to gain access, which can accommodate a 0.038” guidewire. More recently, micropuncture techniques have been popularized, which involve the use of small initial access needles and wires, <21 gauge, with upsizing catheters that ultimately allow placement of a standard guidewire and sheath.
TABLE 29-1 Comparison of Vascular Access Sites for Endovascular Procedures | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
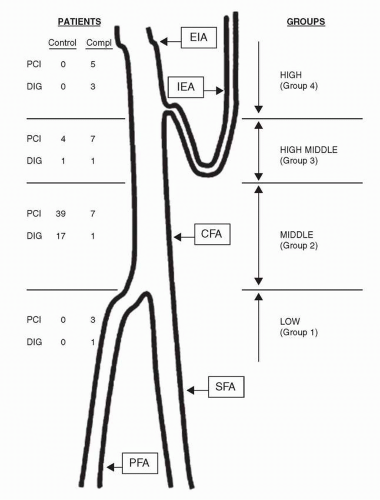
Femoral artery access is optimal when the common femoral artery (CFA) is the site of entry of the needle, guidewire, and sheath. Access sites at or below the CFA are associated with increased rates of pseudoaneurysms and hematomas (2), and limit the ultimate size of a sheath that can be used (Fig. 29-1). Access sites in the external iliac artery (EIA), that is, above the CFA, are problematic in that the EIA is in a retroperitoneal location and can be associated with increased rates of bleeding (2, 3).
Several techniques have been employed to guide femoral artery access, including use of anatomic markers, palpation of the femoral head, fluoroscopy, and ultrasound. While all of the other techniques rely on extrapolating the relationship between the CFA and the head of the femur from fluoroscopy, only ultrasound allows visualization of the CFA and its bifurcation. The CFA bifurcation is usually below the head of the femur, but can be within the silhouette of the femoral head in up to 25% of cases (2). The EIA is usually above the femoral head, but can be within the silhouette of the femoral head in up to 25% of cases (2). The most commonly employed access technique today utilizes fluoroscopy of the femoral head in an AP projection, with a skin access point at the bottom of the femoral head, identified using a surface marker such as a pair of hemostats. A variation of this technique has been proposed involving fluoroscopy just before the needle enters the femoral artery, to confirm that the access site will be in the middle of the femoral head (the highest likelihood of the presence of the CFA) (4).
In addition to minimizing access site complications, access of the CFA is necessary for optimal utilization of VCDs (see below). Three studies recently compared the fluoroscopy technique with an anatomic landmark technique and found no differences in rates of vascular complications between the two methods, although there was a slightly higher rate of high sticks with the landmark technique (5, 6 and 7). Ultrasound-guided femoral artery access has been compared with fluoroscopy-based access, and also did not find any differences in the rates of vascular complications, but did observe a slightly higher rate of high stick with ultrasound-guided access (8). With the increase in the use of large bore sheaths for new devices
(percutaneous ventricular assist devices, transcatheter aortic valves, etc.), the need for CFA access has also increased. Fortunately, imaging of the pelvic vasculature is obtained prior to these procedures, so the location of the CFA relative to the femoral head is available at the time access is needed.
(percutaneous ventricular assist devices, transcatheter aortic valves, etc.), the need for CFA access has also increased. Fortunately, imaging of the pelvic vasculature is obtained prior to these procedures, so the location of the CFA relative to the femoral head is available at the time access is needed.
Brachial and Radial Artery
Brachial artery access is obtained in a similar fashion as femoral artery access. The most common access site is 1 cm proximal to the antecubital fossae, bordered by the medial and lateral epicondyles of the elbow laterally. Because of the development of radial artery catheterization and intervention, brachial artery use as an access site has fallen dramatically. Nonetheless, in selected cases, it provides an alternative access site to the femoral artery, and can accommodate larger sheaths and catheters (up to 8-9 Fr) than can the radial artery.
Radial artery access differs from both brachial and femoral artery access because of the smaller caliber of the vessel, and its propensity for spasm. The radial artery can be used as an access site in up to 95% of patients, in whom a patent palmar arch provides flow to the hand from the ulnar artery independently of a patent radial artery. Interosseous collaterals also provide flow to the hand independent of either radial or ulnar artery flow. The patency of the palmar arch, and thus the “dual blood supply” to the hand, should be determined prior to the procedure. This is documented using the modified Allen’s test, where the blood supply to the hand is assessed while occluding the radial artery. Assessment of the blood flow to the hand includes return of color to the hand after the initial pallor from both radial and ulnar artery occlusion, oximetry, and Doppler flow.
The access site of the radial artery is most commonly obtained 1 cm proximal to the styloid process. An intradermal wheal of 1% lidocaine is used to anesthetize the skin over the access site. In our lab, in an attempt to maximize palpation of the radial artery and to minimize spasm of the vessel, we inject additional lidocaine only when access has been obtained. To gain access, 21-gauge or smaller needles are used, most optimally using a posterior wall technique. Either a bare needle or an Angiocath can be used. Small caliber guidewires capable of being introduced
through the small needle are advanced, and a hydrophilic sheath is inserted. In contrast to brachial and femoral artery access sites, it is important to administer a spasmolytic agent at the time of sheath insertion to minimize radial artery spasm. Additionally, anticoagulation is important to prevent radial artery occlusion. The reader is referred to Chapter 28 for further discussion of radial artery access, anticoagulation, and of anomalous radial artery anatomy.
through the small needle are advanced, and a hydrophilic sheath is inserted. In contrast to brachial and femoral artery access sites, it is important to administer a spasmolytic agent at the time of sheath insertion to minimize radial artery spasm. Additionally, anticoagulation is important to prevent radial artery occlusion. The reader is referred to Chapter 28 for further discussion of radial artery access, anticoagulation, and of anomalous radial artery anatomy.
ACCESS SITE MANAGEMENT
Manual Compression
Since the brachial and femoral arteries are the sole blood supply to the arm and the leg, hemostasis can be achieved by compression of the artery proximal to the access site. After sheath removal for routine diagnostic catheterization, occlusive pressure is usually maintained for 3-5 minutes, followed by subocclusive pressure for another 3-10 minutes. The duration of compression is influenced by multiple factors, including the size of the sheath, extent of anticoagulation, and comorbidities such as chronic kidney disease that affects the propensity to coagulate. Additionally, patient habitus may affect the ability to optimally achieve an appropriate compression force. After PCI, where anticoagulation is always used, timing of sheath removal and manual compression is usually determined by the type of anticoagulant used. For unfractionated heparin, sheath removal and manual compression are usually performed when the activated clotting time (ACT) has fallen below 180 seconds. After low-molecular-weight heparin, sheath removal and manual compression are usually performed 4-6 hours after the last dose of medication. After bivalirudin, the standard is to wait 2 hours and then remove the sheath and apply manual compression. The duration of manual compression after PCI will be longer than that needed for simple diagnostic catheterization, and will be determined by local guidelines.
Manual compression is generally safe and effective, and is still considered by many to be the gold standard for achieving hemostasis. However, manual compression may be associated with patient dissatisfaction issues such as delayed ambulation, local discomfort, and time/personnel needed to achieve hemostasis. Whether manual compression, or alternative methods such as the use of VCDs, becomes the standard for hemostasis will be determined by each lab after weighing the risks and benefits of each method.
Radial artery hemostasis can also be achieved by manual compression. Because of the dual blood supply to the hand, however, attention must be paid to both antegrade and retrograde flow to the radial artery during hemostasis. Additionally, because of the concern for radial artery occlusion, radial sheaths are commonly removed with full anticoagulation present, so hemostasis is achieved over a much longer period than for a typical femoral artery access site. Finally, the technique of perfused hemostasis (hemostasis using a closure band with perfusion documented to the hand by oximetry) has been developed with a lower incidence of radial artery occlusion than with simple compression (9). The reader is referred to Chapter 28 for a more complete discussion of this technique.
FACILITATED MANUAL COMPRESSION
In an effort to reduce the personnel time and effort associated with manual compression, a number of external compression devices have been developed to aid and facilitate manual compression. The most commonly used clamp device has a flat base that lies under the patient’s hip, and a compression arm that can be moved up and down along a vertical stand attached to the base. The tip of the arm has a compression disc that is applied to the lower abdomen/upper groin to compress the femoral artery analogous to handheld pressure. There is a locking system that maintains hemostatic pressure between the base plate and the arm that can be released at the operator’s discretion. While there was initial enthusiasm for the devices, movement of the patient can result in inadvertent slippage of the compression arm and loss of hemostasis. As a result, and because of inconsistent results, these devices have lost favor and are now seldom used.
A more sophisticated external compression device is the Femo-Stop (St. Jude Medical; St. Paul, MN). This consists of a compression disc with an internal balloon that can apply variable compression pressure, depending on the extent of inflation of the tamponading balloon. The device is secured around the patients’ hips by a 6″ mesh band that minimizes movement and helps secure the compression device over the femoral artery. This device is often used in the setting of a rapidly expanding hematoma that cannot be adequately managed with manual compression.
Topical Hemostatic Patches
Hemostatic patches have been used as an adjunct for manual compression for management of femoral access site sheath removal. Use of hemostasis patches have been shown, in small studies, to reduce the time to hemostasis and ambulation compared with manual compression after percutaneous procedures involving femoral artery access (10, 11, 12, 13 and 14). However, only limited data are available examining their efficacy and safety in broader clinical practice (15, 16). The risk of local adverse events following the use of two patches, Chito-Seal (Abbott Vascular Devices; Redwood City, CA) and the Syvek NT Patch (Marine Polymer Technologies, Inc.; Dankers, MA), for access site hemostasis after cardiac catheterization procedures has been reported (16). Neither patch was associated with a lower risk of vascular complications compared with manual compression alone, although there was a trend toward a higher risk of entry-site bleeding with the Syvek patch compared with manual compression.
Topical thrombin has been used for local hemostasis for a variety of surgical and nonsurgical procedures with great success (17, 18). It has been incorporated into a bandage for topical hemostasis, including use for cardiac catheterization procedures. However, the safety and efficacy of thrombin hemostatic patch for access site hemostasis after diagnostic cardiac catheterization and PCI procedures in clinical practice remains unclear.
VASCULAR CLOSURE DEVICES
Types of VCDs
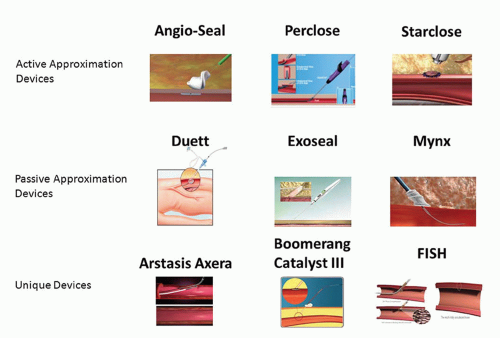
There are several VCDs that have achieved commercial and clinical application. VCDs fall into two broad categories: those that achieve hemostasis by active approximation of the arterial wall at the arteriotomy site and those that achieve hemostasis by passive approximation or tamponade of the arteriotomy site, often termed “extravascular” devices (Fig. 29-2) (4). The three commonly used active approximation devices are the Angio-Seal, Perclose, and StarClose devices, and the commonly used passive approximation devices are the Duett, ExoSeal, and Mynx devices. Each of these devices will be discussed briefly in the following section.
VCDs—Active Approximation
The Angio-Seal device (St. Jude Medical; St. Paul, MN) consists of an intraluminal polylactic-polyglycolic acid polymer anchor (2 × 11 mm) attached to suture over which a collagen sponge is compressed against the external wall of the artery and ultimately secured by a knot, forming an “arteriotomy sandwich” at the access site (19). The anchor is nonthrombogenic, and all of the components are all bioresorbable, with chemically complete bioabsorption occurring after 3 months. The device is introduced with its own delivery sheath and is available in 6 and 8 Fr sizes. It is generally accepted that this device should be used for the 8 or 9 Fr or smaller sheath sizes. The Perclose devices (Abbott Vascular; Redwood City, CA) Proglide and Prostar both achieve percutaneous suture-mediated closure in a fashion analogous to that obtained by direct surgical closure of a vessel (20). The Proglide device deploys two sutures through the anterior wall of the artery, received by the foot plate within the artery and then exteriorized. The device is then removed from the artery leaving a pretied suture knot. Success achieving hemostasis with the Perclose exceeds 90% (21




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree