Cardiomyopathy is a frequent cause of death in patients with Friedreich ataxia (FA), and a characteristic pathological feature is the focal accumulation of iron (Fe) in cardiomyocytes. This restricted localization of the metal contrasts with the diffuse cardiac Fe overload in hemochromatosis and transfusion siderosis. Nevertheless, heart Fe in FA contributes to cardiomyocyte necrosis, inflammation, and scarring as the disease progresses. A putative mechanism of cardiomyopathy in FA is Fe-mediated oxidative damage. Two other transition metals zinc (Zn) and copper (Cu), are diffusely distributed throughout normal hearts and the hearts of patients with FA. The myocardium in FA is also prone to deposits of calcium in the form of scattered concretions. In this study, heart tissues (left and right ventricular walls and ventricular septum) of 23 patients with genetically confirmed FA and 8 normal controls were obtained at autopsy and analyzed for Fe, Zn, Cu, and calcium. The principal assay methods were inductively coupled plasma optical emission spectrometry and plasma mass spectrometry. Total levels of Fe in bulk extracts were not significantly higher than normal, and the concentrations of Zn also remained in the normal range. Cu levels, however, were significantly lower in FA. In conclusion, the decrease of Cu may be important in consideration of the potential benefit of Cu supplements in FA cardiomyopathy.
The mutation in Friedreich ataxia (FA), a pathogenic homozygous guanine-adenine-adenine trinucleotide repeat expansion, leads to a systemic deficiency of frataxin. This small mitochondrial protein is essential for the biogenesis and delivery of iron (Fe)-sulfur clusters, whereas other roles, such as Fe storage and detoxification, are less well defined. FA affects central and peripheral nervous systems, dorsal root ganglia, heart, skeleton, and the endocrine pancreas of children, adolescents, and adults. The most common cause of death in FA is cardiomyopathy, and in some patients, heart disease precedes the neurological manifestations. Frataxin levels in the left ventricular wall (LVW) of FA hearts are at or below the detection limit of an enzyme-linked immunosorbent assay (<15 ng/g wet weight), whereas those in control LVW samples have an average of 235.4 ± 75.1 ng/g wet weight (mean ± SD). The description of Fe excess in cardiomyocytes of FA hearts antedates the discovery of the mutation. Chemical assays of total Fe in bulk extracts of FA hearts do not reveal a net increase, but in situ quantification by X-ray fluorescence shows elevated levels in highly localized, randomly distributed, regions of the heart. In contrast, zinc (Zn) remains normal. The piecemeal Fe excess in the myocardium of patients with FA is unlike the diffuse distribution in Fe overload diseases, such as primary hereditary hemochromatosis and transfusion siderosis. In the present study, Fe, Zn, copper (Cu), and calcium (Ca) were measured in an autopsy series of FA hearts to identify a potential impact of these metals on the pathogenesis of FA cardiomyopathy. Surprisingly, this analysis discovered cardiac Cu deficiency.
Methods
The Institutional Review Board of Veterans Affairs Medical Center (Albany, New York) approved this study. Frozen heart specimens were collected from 23 deceased patients (11 men and 12 women) with FA over a period of 10 years through a tissue donation program supported by Friedreich’s Ataxia Research Alliance (Downingtown, Pennsylvania). Ages of death were 10 to 69 (34.7 ± 15.4 [mean ± SD]). All patients had homozygous guanine-adenine-adenine trinucleotide repeat expansions. Twenty-two had heart failure or arrhythmia during life. Microscopic examination showed cardiomyocyte hypertrophy, endomysial fibrosis, and Fe-positive inclusions in all patients. Eight frozen heart samples from subjects without heart disease (3 men, 5 women; age of death 49.9 ± 11.8 years [mean ± SD]) were obtained from National Disease Research Interchange (Philadelphia, Pennsylvania) for use as a control group. Hearts were thawed under clean room conditions, and samples were dissected from LVW, right ventricular wall (RVW), and ventricular septum (VS) by stainless steel instruments. For one FA heart, only the LVW was available for analysis. Formalin-fixed, paraffin-embedded, tissue samples of the 23 FA hearts were also available. Sections were stained by hematoxylin and eosin, and by Perls’s Fe stain.
All elemental determinations in heart tissue were performed in the Laboratory of Inorganic and Nuclear Chemistry at the New York State Department of Health (Wadsworth Center), Albany, New York. Frozen tissue samples were thawed, weighed, and freeze-dried to constant weight before being digested in concentrated nitric acid with microwave-assisted heating (MARS5; CEM Corporation, Matthews, North Carolina). Elemental measurements were obtained using inductively coupled plasma optical emission spectrometry (ICP-OES; Perkin Elmer Optima 5300 DV; Shelton, Connecticut). The samples were analyzed a second time using single quadrupole inductively coupled plasma mass spectrometry (ICP-MS; Perkin Elmer DRC II; and NexION 300D, Shelton, Connecticut). The detection limit for Cu by ICP-OES (4 ng/g) was insufficient for this determination. Therefore, all Cu data were based on ICP-MS measurements. All metal levels were expressed as μg/g wet weight to compare with other data in the studies.
Tissue concentrations of each metal were analyzed separately by repeated measures analysis of variance with a between-group effect of disease (2 levels, FA and normal), a within-patient myocardial location effect (3 levels, LVW, RVW, VS), and their interaction. Multiple comparisons were by Tukey’s test. There was no evidence that Fe, Zn, or Cu distributions were other than normal (Anderson–Darling test) nor was there any indication of heteroscedasticity (all SD were well within an order of magnitude). For Ca, the patients with FA demonstrated non-normal distributions in all 3 myocardial locations (p <0.01; Anderson–Darling test) due to pronounced upward skew. The skew could not be corrected by transformation, and a Mann–Whitney test was therefore used for each of the 3 myocardial locations separately. Statistical software included JMP (version 10.0 General Linear Model; SAS Statistical Software, Cary, North Carolina) and Minitab (version 17, Mann–Whitney tests; Minitab, Inc., State College, Pennsylvania).
Results
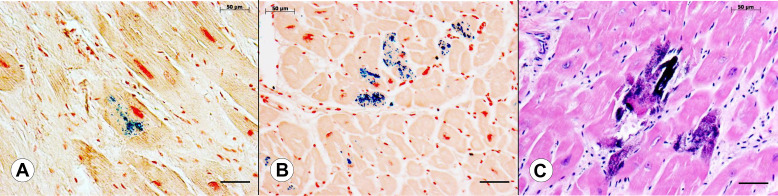
Figure 1 shows the appearance of Fe in sections of LVW from 2 different patients with FA and Ca in a third patient with FA. Accumulation of Fe ranges from small granules in a single cardiomyocyte ( Figure 1 ) to involvement of several adjacent fibers ( Figure 1 ). In addition to abnormal Fe, Figure 1 also displays the characteristic fibrosis of the heart in FA cardiomyopathy. The section stained by hematoxylin and eosin ( Figure 1 ) reveals intensely basophilic material that may be interpreted as calcification. These concretions are also Fe-positive.
Results for Fe, Cu, Zn, and Ca in FA and control hearts are displayed in Tukey box-and-whisker plots ( Figure 2 ). Pooled Fe levels in normal controls were 53.1 ± 14.6 μg/g (mean ± SD) compared with 71.3 ± 20.6 μg/g (mean ± SD) in FA. The higher levels in patients with FA did not achieve statistical significance (main effect of group; p = 0.064). There was a significant effect, however, of location in FA (main effect of location; p = 0.001) with RVW Fe levels significantly lower than LVW and VS, which did not differ from each other ( Figure 2 ). There was no indication that disease had differing effects in the 3 locations (interaction effect; p = 0.94; Figure 2 ).
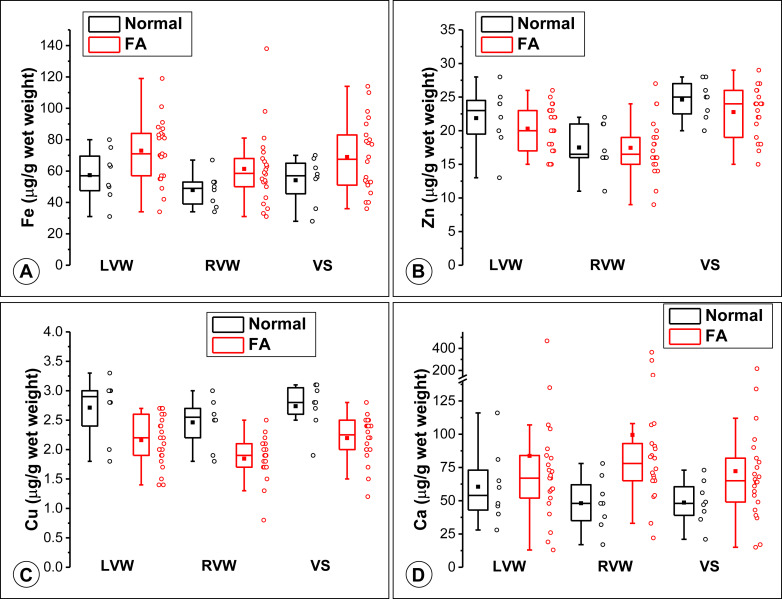
Pooled Zn levels were not significantly different, averaging 21.5 ± 2.9 μg/g (mean ± SD) in FA and 23.1 ± 3.4 μg/g (mean ± SD) in normal hearts ( Figure 2 ), indicating that the disease had no effect on the levels of this metal (group effect; p = 0.36). There was, however, a significant effect of location (p <0.001; Figure 2 ): Zn was highest in VS, lowest in RVW, and all locations were significantly different from one another. Considering patients with FA only, RVW Zn was significantly lower than LVW Zn (p = 0.03) and VS (p <0.001), but LVW and VS were not different from one another (p = 0.06). There was no indication that disease had differing effects in the 3 locations (interaction effect, p = 0.52).
For Cu, there was a statistically significant effect of disease (p <0.001) and location (p <0.001) without an interaction effect (p = 0.90). Cu levels across the 3 locations in normal subjects were 2.72 ± 0.42 μg/g (mean ± SD) compared with 2.18 ± 0.40 μg/g (mean ± SD) in patients with FA. In FA and controls, overall LVW and VS Cu levels were not different, but RVW Cu was significantly lower than both LVW Cu and VS Cu ( Figure 2 ). Considering patients with FA only, RVW Cu was significantly lower than LVW Cu (p = 0.01) and VS (p = 0.003), but LVW and VS were not different from one another (p = 0.99). There was no significant interaction effect of disease and location on Cu, indicating that the effect of disease was not different in the 3 myocardial locations.
Ca demonstrated upwardly skewed levels in patients with FA because of several very high outliers ( Figure 2 ). Mann–Whitney tests applied to the samples from the 3 regions separately showed no difference in LVW (p = 0.38; median: 54.0 μg/g, interquartile range [IQR]: 41.5 to 77.0 μg/g in normal subjects; and median: 67 μg/g, IQR: 52.0 to 84.0 μg/g in FA) or VS (p = 0.08; 48 μg/g, IQR: 37.5 to 62.8 μg/g in normal subjects; and median: 65 μg/g, IQR: 47.5 to 84 in FA). RVW Ca was significantly higher in FA (p = 0.008; median: 48 μg/g, IQR: 33.5 to 65.5 μg/g in normal subjects; and 78 μg/g, IQR: 65 to 96.5 μg/g in FA).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


