Morphologic abnormalities of the left ventricular (LV) outflow tract generally result in one or more of the following pathophysiologic states: obstruction, regurgitation, and aneurysmal dilation of the proximal aorta. Obstruction is the most prevalent problem, whereas the other two are rare in children. Known generally as aortic stenosis (AS), LV outflow tract obstruction occurs in approximately 5 per 10,000 live births, represents 5% to 8% of all congenital heart diseases, and usually ranks as the sixth or seventh most common lesion. Among all patients with LV outflow tract obstruction, valvar AS is the most common subgroup (with a frequency of 60% to 75%), followed by subvalvar AS (8% to 30%) and supravalvar AS (1% to 2%). In addition, a bicuspid aortic valve (AoV) with or without stenosis occurs in up to 2% of the general population, thereby representing the most common congenital heart lesion.
CLINICAL PRESENTATION
LV outflow tract obstruction is associated with a pressure-overloaded left ventricle, often accompanied by progressive LV hypertrophy and fibrosis. Patients with LV outflow tract obstruction generally present with a systolic murmur whose frequency and intensity are determined by the degree of obstruction. Occasionally, a systolic click is heard in the setting of an abnormal AoV. Symptoms in children with LV outflow tract obstruction are rare, although severe obstruction can be associated with syncope, chest pain, and/or exercise intolerance. Very rarely, older children with significant obstruction will present with signs of congestive heart failure as severe LV hypertrophy results in progressive diastolic and systolic dysfunction. Many patients with the rare subgroup of supravalvar AS also have Williams syndrome with its characteristic features of infantile hypercalcemia, failure to thrive, elfin-like facial abnormalities, and mental retardation. The electrocardiogram may show increased left-sided voltages with ST-T wave changes consistent with LV hypertrophy. The heart is generally normal in size on the chest radiograph, although the ascending aorta may be dilated.
Significant LV outflow tract obstruction in the newborn period often involves severe LV dysfunction, mitral regurgitation, and increased left-to-right shunting across the foramen ovale, all contributing to compromised cardiac output. These infants generally present with signs and symptoms of congestive heart failure and shock. In cases of critical AS, cardiac output across the LV outflow tract is inadequate, and ductal patency must be maintained with prostaglandin E1 infusion so that the right-to-left flow across the ductus augments cardiac output for sufficient tissue oxygen delivery. The electrocardiogram may show increased right-sided and/or left-sided voltages with ST-T wave changes. The heart is usually enlarged on the chest radiograph, and pulmonary edema may be present.
Aortic regurgitation (AR) is associated with a volume-overloaded left ventricle. In contrast to the ventricular hypertrophy which results from pressure overload, volume overload results in ventricular dilation with compensatory hypertrophy (compensated AR). However, the heart’s ability to sustain hypertrophy eventually fails (decompensated AR) and LV wall stress increases, eventually leading to irreversible ventricular dysfunction and poor contractility. A diastolic murmur is generally heard on physical examination. Significant AR is usually associated with a hyperdynamic LV impulse and prominent peripheral pulses. These patients may begin to show signs and symptoms of congestive heart failure. The electrocardiogram will usually show increased left-sided voltages with ST-T wave changes, and cardiomegaly may be seen on the chest radiograph.
Aneurysmal dilation of the aortic root and/or ascending aorta is generally discovered because of its association with a bicuspid AoV, or a specific genetic syndrome or disease prompts a clinician to perform an echocardiogram or another diagnostic study. Rarely, a pediatric patient with aneurysmal dilation of the ascending aorta has an aortic dissection or rupture and presents with acute chest or abdominal pain, shock, or sudden death. Occasionally, a sinus of Valsalva aneurysm can rupture without catastrophic results, resulting primarily in a significant left-to-right shunt from the aorta into a right-sided chamber with consequent congestive heart failure. The chest radiograph generally shows the enlarged aortic root or ascending aorta, and cardiomegaly may be present in the setting of significant left-to-right shunting.
NORMAL ANATOMY
The normal LV outflow tract can be divided into three anatomic segments: the subaortic region, the AoV, and the proximal aorta (Fig. 14.1). In utero, the conus or infundibulum represents the subarterial muscular chamber separating the atrioventricular valve from the corresponding semilunar valve in both the right ventricular (RV) and LV outflow tracts. In normal fetal hearts, the subaortic conus regresses, resulting in varying degrees of fibrous continuity between the mitral valve (MV) and the AoV (Fig. 14.2). The area of fibrous continuity is often referred to as the mitral-aortic intervalvular fibrosa, a structure that originates from the primordial left ventriculoinfundibular fold and can be elongated in cases of subvalvar AS and tetralogy of Fallot. It is usually measured as the shortest distance from the anterior mitral leaflet to the base of the noncoronary or left coronary leaflet of the AoV. Within the LV outflow tract, the anterior mitral leaflet represents the posterior boundary of the subaortic region and the muscular ventricular septum represents the anterior boundary (see Fig. 14.2).
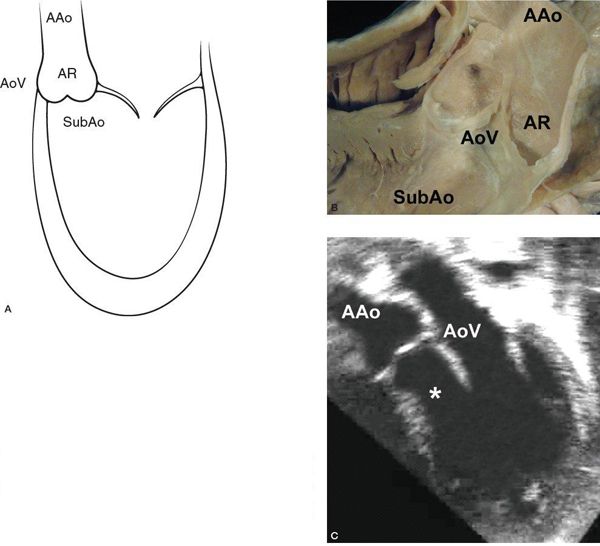
Figure 14.1. The anatomic segments of the left ventricular outflow tract. Includes the subaortic region (SubAo, asterisk), the aortic valve (AoV), and the ascending aorta (AAo), as depicted in (A) an illustration, (B) a pathology specimen, and (C) an apical long-axis view. AR, aortic root. (B, With permission from Prof. Robert H. Anderson, Institute of Child Health, London, United Kingdom.)
The normal AoV is a three-dimensional structure that involves three leaflets attached to the aortic root and supporting ventricular muscle in a semilunar or crown-like fashion (see Figs. 14-1B and 14-2A). The leaflets are separated by three commissures, which are lines of apposition between the leaflets extending from the center of the valve to the periphery (Fig. 14.3) (Video 14.1) and from the area of the ventriculoarterial junction to the sinotubular junction (the junction between the aortic root and proximal ascending aorta). An important and somewhat problematic issue in AoV morphology is the concept of the “aortic annulus,” a diagnostician construct that does not correspond to a true anatomic structure. Although an anatomic ventriculoarterial junction can usually be identified pathologically, the basal attachments of the semilunar leaflets actually extend beyond this ring into the supporting ventricular muscle (see Fig. 14.2), thereby confounding the use of the most proximal or basal attachment of the semilunar valve leaflets to define the “aortic annulus” (Fig. 14.4). In other words, the “aortic annulus” is not synonymous with the anatomic ventriculoarterial junction along the LV outflow tract. In addition, this ventriculoarterial ring does not fully support the AoV because the leaflets are attached or hinged within the entire aortic root up to the level of the sinotubular junction.
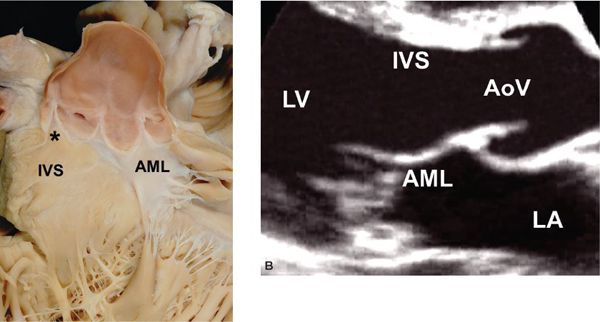
Figure 14.2. Fibrous continuity between the mitral valve and the aortic valve. A: Pathology specimen showing the anatomic ventriculoarterial junction (asterisk). B: Parasternal long-axis view. AML, anterior mitral leaflet; AoV, aortic valve; IVS, interventricular septum; LA, left atrium; LV, left ventricle. (A, With permission from Prof. Robert H. Anderson, Institute of Child Health, London, United Kingdom.)
The components of the proximal aorta include the aortic root and ascending aorta, structures that meet at the sinotubular junction (see Fig. 14.4). Because the sinotubular junction represents the most distal attachments of the AoV leaflets within the aortic root, it may be somewhat confusing to label an anomaly at the sinotubular junction a supravalvar anomaly because it often involves the distal segment of the AoV. Nevertheless, the walls of the aortic root and the ascending aorta are composed primarily of vascular smooth muscle cells and extracellular matrix proteins secreted by the cells. The most common component of the aortic wall is elastin, a protein that now appears to play a significant role in the morphogenesis and homeostasis of the arterial vessel walls. In addition, it appears to be involved in the development of supravalvar obstruction and aneurysmal dilation of the proximal aorta.
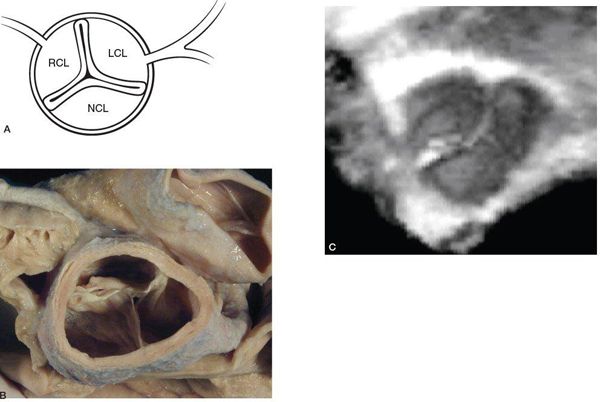
Figure 14.3. Cross-sectional view of the aortic valve with three leaflets separated by three commissures. Depicted in (A) an illustration, (B) a pathology specimen, and (C) a three-dimensional echocardiographic image. LCL, left coronary leaflet; NCL, noncoronary leaflet; PV, pulmonary valve; RCL, right coronary leaflet. (B, With permission from Prof. Robert H. Anderson, Institute of Child Health, London, United Kingdom.)
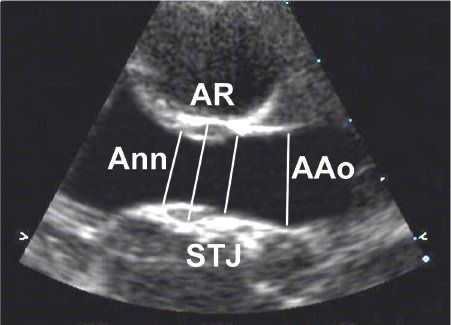
Figure 14.4. Parasternal long-axis view of the proximal aorta. Diameters are measured at the levels of the aortic “annulus” (Ann), aortic root (AR), sinotubular junction (STJ), and ascending aorta (Aao).
ABNORMAL ANATOMY AND COMMON ASSOCIATIONS
Valvar Aortic Stenosis
Etiologies for valvar AS are listed in Table 14.1. The most common abnormality of AoV morphology is the bicuspid AoV, in which two of the three leaflets are fused or one of the commissures between adjacent leaflets is underdeveloped (also known as a raphe) (Fig. 14.5) (Video 14.2). A true bicuspid AoV with only two leaflets is a rare phenomenon. In most cases of a bicuspid AoV, the size of the combined leaflet (secondary to fusion or underdevelopment of a commissure) is larger than the unaffected leaflet, although it is rarely exactly twice the size of the unaffected leaflet. This latter finding suggests that the lesion is a developmental problem of the entire valve rather than simple fusion of two of the three leaflets. Among all patients with a bicuspid AoV, fusion or underdevelopment occurs most commonly at the intercoronary commissure between the right and left coronary leaflets (with a frequency of 70% to 86%) followed by the commissure between the right and noncoronary leaflets (12% to 28%) and the commissure between the left and noncoronary leaflets (very rare) (Fig. 14.6) (Video 14.3). In addition, the rate of AS progression appears to be higher with underdevelopment of the intercoronary commissure. Common associations with a bicuspid AoV include aortic coarctation (which occurs more frequently with intercoronary commissural underdevelopment) or interrupted aortic arch, subvalvar AS, ventricular septal defect (VSD), coronary anomalies (such as a displaced coronary ostium or a shortened left coronary artery), Turner syndrome, aortic dilation or aneurysm formation (with the increased risk for aortic dissection and rupture) (Fig. 14.7) (Video 14.4), and endocarditis (Fig. 14.8, Table 14.2) (Video 14.5).
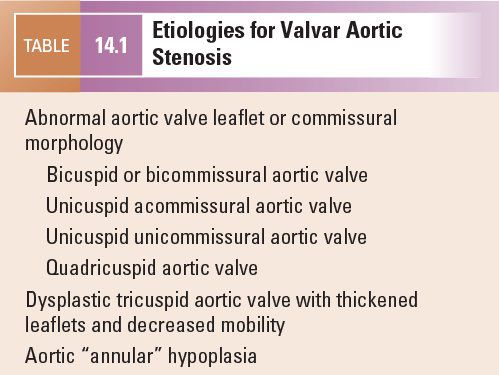
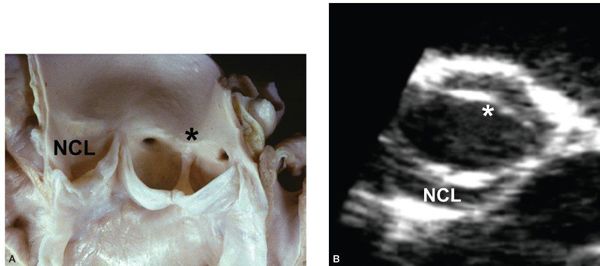
Figure 14.5. Bicuspid aortic valve with fusion or underdevelopment of the intercoronary commissure (asterisks). Depicted in (A) a pathology specimen and (B) a parasternal short-axis view. NCL, noncoronary leaflet. (A, With permission from Prof. Robert H. Anderson, Institute of Child Health, London, United Kingdom.)
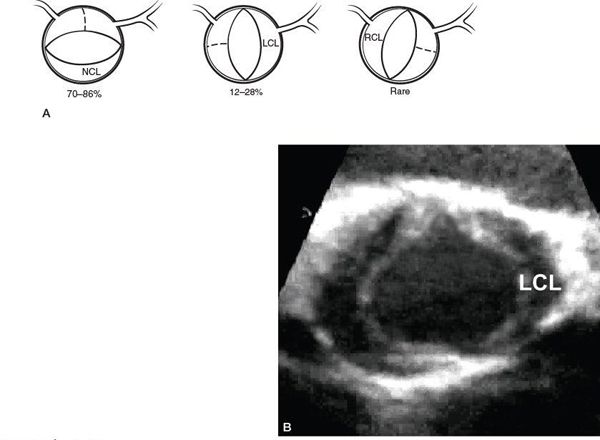
Figure 14.6. Bicuspid aortic valve. A: Illustration of the three subtypes of a bicuspid aortic valve and their relative frequencies. B: Parasternal short-axis view of a bicuspid aortic valve with fusion or underdevelopment of the commissure between the right coronary and noncoronary leaflets. LCL, left coronary leaflet; NCL, noncoronary leaflet; RCL, right coronary leaflet.
Figure 14.7. Parasternal long-axis view of a bicuspid aortic valve with a dilated ascending aorta. AAo, ascending aorta; BAV, bicuspid aortic valve.
The most common AoV morphology in neonatal valvar AS is a unicuspid unicommissural AoV where the only open commissure is located between the left and noncoronary leaflets; in addition, a small eccentric valvar orifice is usually seen (Fig. 14.9) (Video 14.6). Occasionally, the leaflets are thickened and poorly formed with decreased mobility (Fig. 14.10) (Video 14.7). These cases are often associated with a small aortic root and ascending aorta. The left ventricle may be small and hypertrophied with endocardial fibroelastosis (see Fig. 14.9E), or it may be dilated with poor systolic function, mitral regurgitation, and left atrial dilation.
Subvalvar Aortic Stenosis
Because subvalvar AS is rarely diagnosed in utero or in the newborn period, many have suggested that this lesion is an acquired heart disease rather than a congenital one. Nevertheless, it is a progressive disease whose postoperative recurrence rate is as high as 33%. The mechanisms for the development of subvalvar AS, as suggested by Cape and colleagues, are listed in Table 14.3. The associated LV outflow tract morphologic abnormalities include a small aortic annulus and root, a steep aortoseptal angle, abnormal LV muscle bundles, a prominent anterolateral muscle bundle of Moulaert, elongation of the mitral-aortic intervalvular fibrosa, MV pathology, and protrusion of aneurysmal tricuspid valve tissue or membranous septum into the LV outflow tract. Subvalvar AS can occur in isolation or with several common associations (Table 14.4).
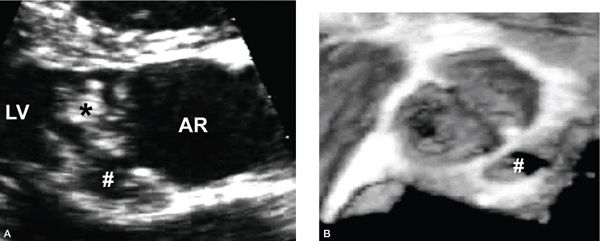
Figure 14.8. Bicuspid aortic valve with endocarditis involving a vegetation adherent to the valve (asterisk) as well as a leftward posterior aortic root abscess (hashed). Depicted in (A) a parasternal long-axis view and (B) a three-dimensional echocardiographic short-axis view. AR, aortic root; LV, left ventricle.
In the absence of a VSD or other cardiac associations, subvalvar AS most frequently presents as a discrete fibrous or fibromuscular shelf extending from the ventricular septum just below the AoV (Fig. 14.11) (Video 14.8). Occasionally, the shelf extends to the anterior mitral leaflet in a diaphragmatic fashion (Fig. 14.12) (Video 14.9). Rarely the muscular component is so extensive that it creates a tunnel-like LV outflow tract with significant subaortic obstruction (Fig. 14.13) (Video 14.10). This is distinct from the more diffusely hypertrophied ventricular septum and dynamic subaortic obstruction associated with hypertrophic cardiomyopathy.
In the presence of a VSD, the most significant type of subaortic obstruction results from posterior deviation of the conal or infundibular septum (Fig. 14.14) (Video 14.11). This lesion is often associated with aortic coarctation or an interrupted aortic arch. Another type of potential subvalvar AS with a VSD involves the presence of an endocardial fold or a fibromuscular ridge at the crest of the muscular septum (Fig. 14.15) (Video 14.12). Last, abnormal MV attachments to the ventricular septum, particularly in the setting of unrepaired or repaired atrioventricular canal defects, can also cause subaortic obstruction (Fig. 14.16) (Video 14.13).
An important and not uncommon association with subvalvar AS involves the development of AR, which can progress in children and result in the need for early surgical intervention (Fig. 14.17) (Video 14.13B). The mechanism for AR presumably involves AoV damage secondary to the high-velocity jet coursing through the subaortic region. In addition, the abnormal fibromuscular tissue in subvalvar AS may be continuous with the base of the AoV, potentially disrupting the normal support for the AoV leaflets. It is important to note, however, that AR associated with subvalvar AS in adults is not usually a progressive problem and does not necessarily suggest a need for surgical intervention.
Supravalvar Aortic Stenosis
Although supravalvar AS is usually associated with Williams syndrome, it can also present as an autosomal dominant lesion in a familial form or as a sporadic idiopathic disorder. Supravalvar AS is in fact an elastin arteriopathy resulting from the mutation or deletion of the elastin gene on chromosome 7. There are three anatomic subtypes of supravalvar AS: the hourglass type, which is the most common subtype and frequently involves dilation of the aortic root and the ascending aorta distal to the narrowing (Fig. 14.18) (Video 14.14); the diaphragmatic or membranous type (Fig. 14.19) (Video 14.15); and the tubular type, which is quite rare and involves diffuse hypoplasia of the ascending aorta (Table 14.5). Common associations include abnormalities of the AoV, the coronary arteries (e.g., coronary artery dilation, intrinsic coronary thickening and ostial stenosis, and ostial occlusion or entrapment by a tethered AoV leaflet) (Fig. 14.20), aortic arch branches, and branch pulmonary arteries (Table 14.6).
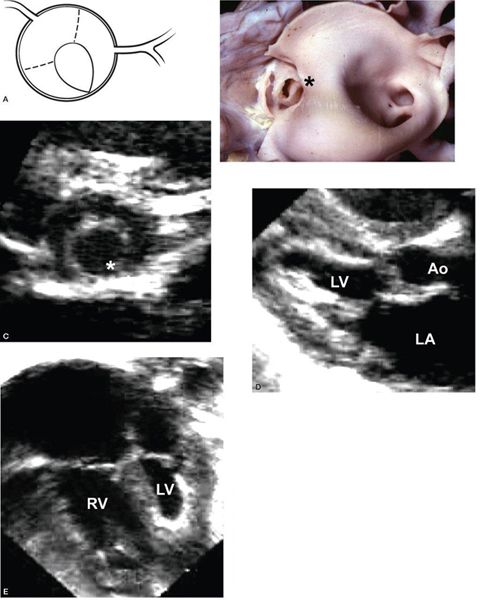
Figure 14.9. Unicuspid unicommissural aortic valve. The only open commissure is located between the left coronary and noncoronary leaflets (asterisks); the aortic valve leaflets are abnormally thickened; and the left ventricle is small with endocardial fibroelastosis, as depicted in (A) a cross-sectional illustration of the aortic valve, (B) a pathology specimen, (C) a parasternal short-axis view, (D) a parasternal long-axis view, and (E) an apical four-chamber view. Ao, aorta; LA, left atrium; LV, left ventricle; RV, right ventricle. (B, With permission from Prof. Robert H. Anderson, Institute of Child Health, London, United Kingdom.)
Aortic Regurgitation
Aortic regurgitation (AR), defined somewhat loosely as diastolic flow from the aorta into the left ventricle, is rare in childhood, especially in isolation. Congenital etiologies for functional AR include a stenotic or nonstenotic bicuspid AoV (Fig. 14.21) (Video 14.16); other abnormalities of AoV leaflet or commissural morphology; an aortico-LV tunnel (Fig. 14.22) (Video 14.17); absence of one or more AoV leaflets; a fibrous band between an AoV leaflet and the aortic root wall; a coronary-cameral fistula into the left ventricle; and a ruptured left sinus of Valsalva aneurysm into the left ventricle (Table 14.7). Other associations with AR include a membranous VSD or a doubly-committed subarterial VSD with AoV prolapse into the defect secondary to the Venturi phenomenon (Fig. 14.23) (Video 14.18), subvalvar AS, neonatal Marfan syndrome with concurrent aortic root dilation, repaired truncus arteriosus, and tetralogy of Fallot (Table 14.8). In addition, AR occurs commonly with a ruptured sinus of Valsalva aneurysm, secondary to disruption of the normal AoV leaflet support within the aortic root (Fig. 14.24) (Video 14.19). Acquired etiologies include endocarditis (Fig. 14.25) (Video 14.20), rheumatic fever, and surgical valvotomy or transcatheter balloon valvotomy for valvar AS (Fig. 14.26, Table 14.9) (Video 14.21).
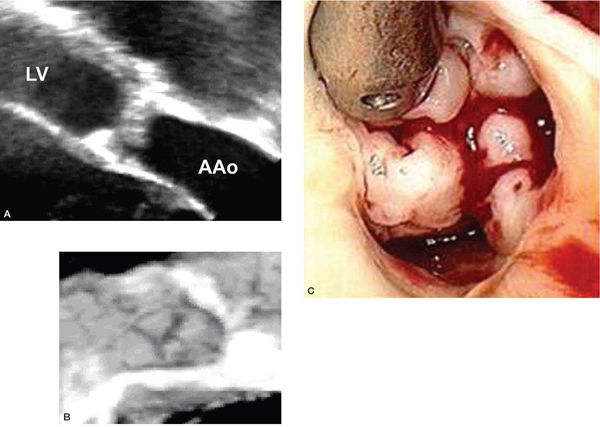
Figure 14.10. Thickened and dysplastic aortic valve leaflets. Depicted in (A) a transesophageal view along a plane oriented at approximately 120 degrees, (B) a three-dimensional short-axis view, and (C) an intraoperative photograph.
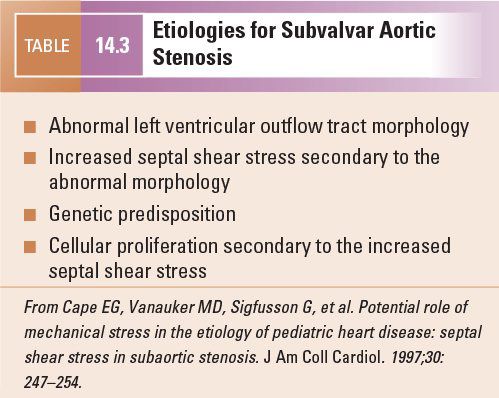
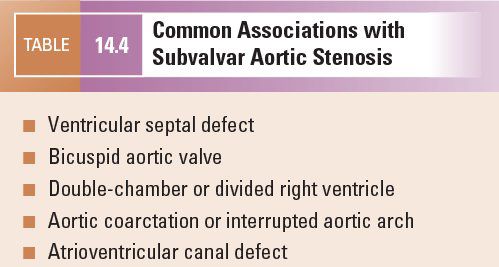
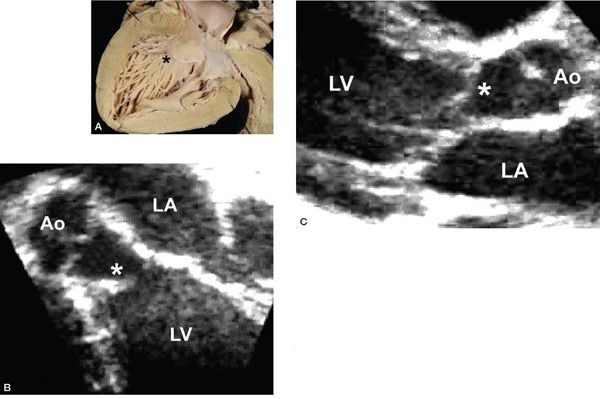
Figure 14.11. Subaortic fibromuscular ridge (asterisks). Depicted in (A) a pathology specimen, (B) an apical long-axis view, and (C) a parasternal long-axis view. Ao, aorta; LA, left atrium; LV, left ventricle. (A, With permission from Prof. Robert H. Anderson, Institute of Child Health, London, United Kingdom.)
Aneurysm of the Proximal Aorta
Most aortic root aneurysms involve the right sinus of Valsalva, followed by the noncoronary sinus and then the left sinus (Fig. 14.27) (Video 14.22). Aneurysm formation in the aortic root and aneurysmal dilation of the ascending aorta (see Fig. 14.7) (Video 14.4) occur when structural weakness and thinning of the aortic wall result from smooth muscle cell loss and extracellular matrix disruption. Elastin production and maintenance appear to be as important in the development of aortic aneurysms as in the arteriopathy associated with supravalvar AS. For example, Marfan syndrome results from mutations in the fibrillin gene on chromosome 15. The fibrillin protein is necessary in the formation of elastin and other components of the extracellular matrix. In addition, it may inhibit the availability and effects of the cytokine transforming growth factor-β and endogenous matrix metalloproteinases, both of which are thought to be involved in the degradation of elastin and other components of the extracellular matrix. Common associations with aneurysmal dilation of the proximal aorta include Marfan syndrome, Loeys-Dietz syndrome, Ehlers-Danlos syndrome, Turner syndrome, a bicuspid AoV, systemic hypertension, and coarctation (Table 14.10). It is important to recognize that the term “poststenotic dilation” of the aorta is no longer appropriate in light of the intrinsic aortopathy associated with valvar AS. In addition, the degree of aortic dilation does not usually correlate to the severity of the stenosis.
APPROACH TO TRANSTHORACIC ECHOCARDIOGRAPHY
Valvar Aortic Stenosis
The transthoracic echocardiogram of a patient with valvar AS must include evaluation of several key features (Table 14.11).
Leaflet and Commissural Morphology
The number and relative sizes of the leaflets and commissures are best evaluated with cross-sectional views of the AoV in parasternal short-axis views. The raphe of a fused or underdeveloped commissure is usually difficult to distinguish from a normal commissure when the AoV is viewed in its closed position during diastole. However, during systole when the AoV is open, the affected commissure remains visible as an echogenic line from the leaflet edge to the aortic wall. Occasionally, there is only partial underdevelopment of a commissure such that the separation between two leaflets occurs but does not extend to the aortic wall (Fig. 14.28) (Video 14.23). When fusion or underdevelopment occurs at the intercoronary commissure in a bicuspid AoV, the AoV opening during systole has a horizontal orientation in parasternal short-axis views (see Fig. 14.5B) (Video 14.2C). In contrast, fusion or underdevelopment of the commissure between the right and noncoronary leaflets results in a more vertical orientation of the AoV opening in the same views (see Fig. 14.6B) (Video 14.3B). In a unicuspid or unicommissural AoV, the opening is usually slit-like in appearance and almost always located at the commissure between the left and noncoronary leaflets (see Fig. 14.9) (Video 14.6A). Acommissural valves usually have a more centrally located orifice. Apical and parasternal long-axis views are very useful in characterizing the AoV leaflets. They may be thickened, and the degree of thickening may vary along the length of the leaflet with thicker edges giving it a “lumpy” appearance (see Figs. 14.9D and 10A) (Videos 14.3A, 14.6B, and 14.7A). In addition, the leaflets usually dome in systole, secondary to the restrictive lateral mobility caused by incomplete commissural separation along the distal zone of apposition (which is located more distally near the sinotubular junction).
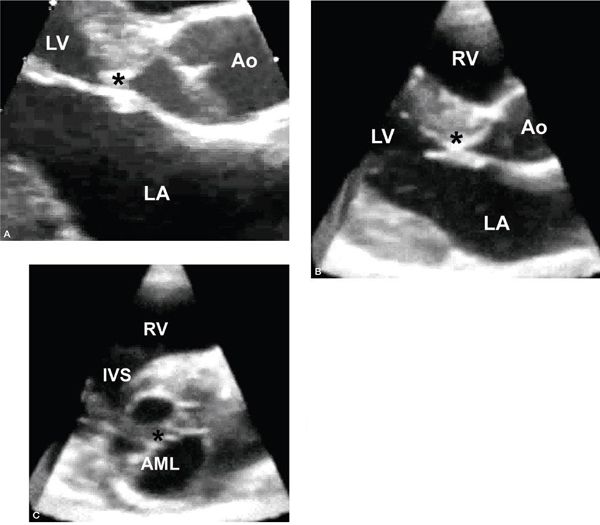
Figure 14.12. Subaortic fibromuscular shelf extending to the anterior mitral leaflet (asterisks). Depicted in (A) a parasternal long-axis view, (B) a three-dimensional long-axis view, and (C) a three-dimensional short-axis view with display of the crescentic shelf adjacent to the anterior mitral leaflet and the diaphragm-like obstruction along the subaortic region. AML, anterior mitral leaflet; Ao, aorta; IVS, interventricular septum; LA, left atrium; LV, left ventricle; RV, right ventricle.
Degree of Obstruction
Continuous-wave Doppler interrogation across the AoV is best performed in apical, right sternal border, and suprasternal views and can provide information regarding the maximum instantaneous gradient and the mean gradient across the AoV. Every effort should be made to align the Doppler beam with the high-velocity jet across the stenotic AoV. It is well known that discrepancies usually occur between the maximum instantaneous gradient measured by echocardiography and the peak-to-peak gradient measured by catheterization, with the former measurement almost always higher than the latter one. These discrepancies result partly from a phenomenon called pressure recovery. Echocardiographic assessment of maximum instantaneous gradients occurs at the vena contracta, the volume of space in the aorta immediately distal to a stenotic AoV where blood flow velocity, kinetic energy, and the pressure drop are highest (Fig. 14.29). The flow velocity normally decreases farther downstream as kinetic energy is converted back to potential energy and pressure is recovered (thereby decreasing the pressure difference between the subaortic region and the more distal ascending aorta). Because the peak-to-peak gradient involves direct pressure measurement more distally in the ascending aorta, pressure recovery may or may not play a role in the arterial pressure. In cases of severe stenosis, the high-velocity jet extends farther into the ascending aorta, and pressure recovery is less influential; in this situation, the discrepancies are less pronounced. On the contrary, the extent of the high-velocity jet is relatively short and pressure recovery occurs more proximally in the ascending aorta with mild stenosis, and the discrepancies in this situation become more apparent.
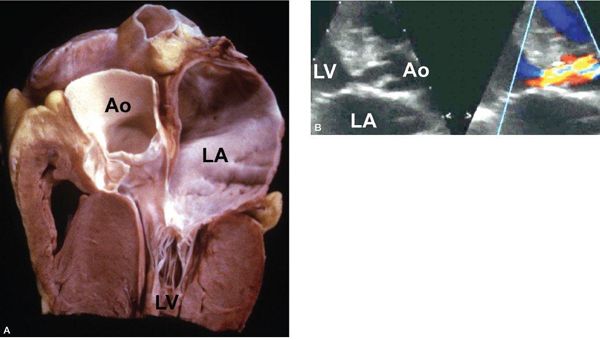
Figure 14.13. Marked septal hypertrophy along the left ventricular outflow tract resulting in significant subaortic stenosis. Depicted in (A) a pathology specimen and (B) a parasternal long-axis view. Ao, aorta; LA, left atrium; LV, left ventricle. (A, With permission from Prof. Robert H. Anderson, Institute of Child Health, London, United Kingdom.)
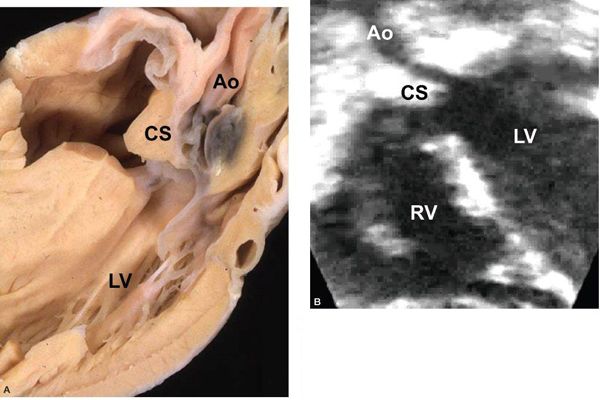
Figure 14.14. Conoventricular septal defect and posterior deviation of the conal septum into the subaortic region. Depicted in (A) a pathology specimen and (B) an apical long-axis view. Ao, aorta; CS, conal septum; LV, left ventricle; RV, right ventricle. (A, With permission from Prof. Robert H. Anderson, Institute of Child Health, London, United Kingdom.)
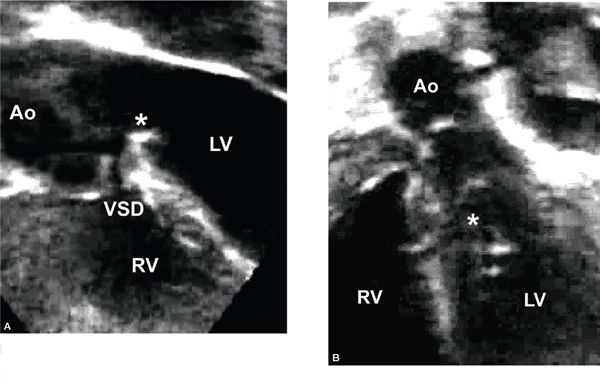
Figure 14.15. Apical long-axis view. Ventricular septal defect (VSD), a fibrous ridge at the crest of the ventricular septum (asterisk), and significant aortic overriding. Ao, aorta; LV, left ventricle; RV, right ventricle.
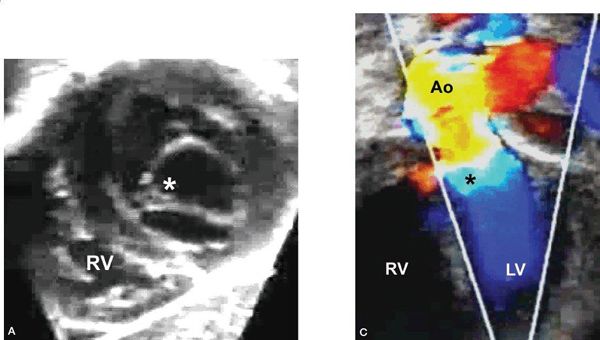
Figure 14.16. Repaired transitional atrioventricular canal defect. Residual cleft mitral valve and subaortic stenosis secondary to the septal attachments of the cleft mitral valve (asterisks), as depicted in (A) a subcostal short-axis view, (B) an apical long-axis view, and (C) an apical long-axis view with color mapping. Ao, aorta; LV, left ventricle; RV, right ventricle.
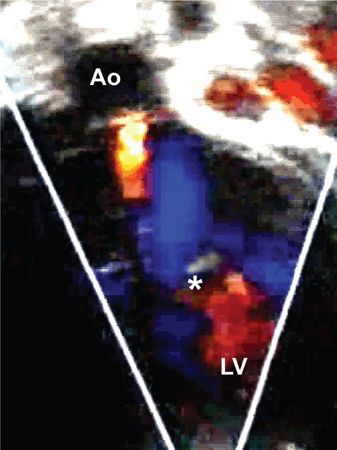
Figure 14.17. Apical long-axis view. Color Doppler from the patient in Figure 14.16 showing mild aortic regurgitation. Septal attachments of the cleft mitral valve (asterisk). Ao, aorta; LV, left ventricle.
Because determination of the degree of valvar AS and the thresholds for intervention are based primarily on catheterization data using peak-to-peak gradients, these discrepancies have confounded the use of echocardiographic data in clinical decision making for these patients. Many prefer the use of Doppler mean gradients to classify valvar AS because these values correlate more closely with catheterization-derived mean gradients. Others accept the limitations associated with using maximum instantaneous gradients, and they use thresholds of 25 to 30 mm Hg to distinguish mild from moderate stenosis and 50 to 60 mm Hg to distinguish moderate from severe stenosis. A recent study by Vlahos and colleagues reveals that catheterization-derived peak-to-peak gradients are overestimated by maximum Doppler gradients and underestimated by mean Doppler gradients from apical and high parasternal views. A maximum Doppler gradient from high parasternal windows less than 55 mm Hg predicts no intervention, whereas predictors of intervention include a maximum Doppler gradient from high parasternal windows greater than 90 mm Hg, a mean Doppler gradient from apical windows greater than 50 mm Hg, and an average of the maximum Doppler gradients from the two views greater than 70 mm Hg. The study concludes that the best predictor of the catheterization-derived gradient is likely an average of the maximum Doppler gradients from apical and high parasternal views.
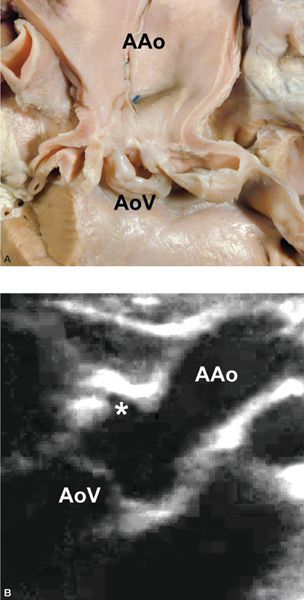
Figure 14.18. Hourglass type of supravalvar aortic stenosis. High origin of the right coronary artery (asterisk), as depicted in (A) a pathology specimen and (B) a right sternal border long-axis view. AAo, ascending aorta; AoV, aortic valve. (A,
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


