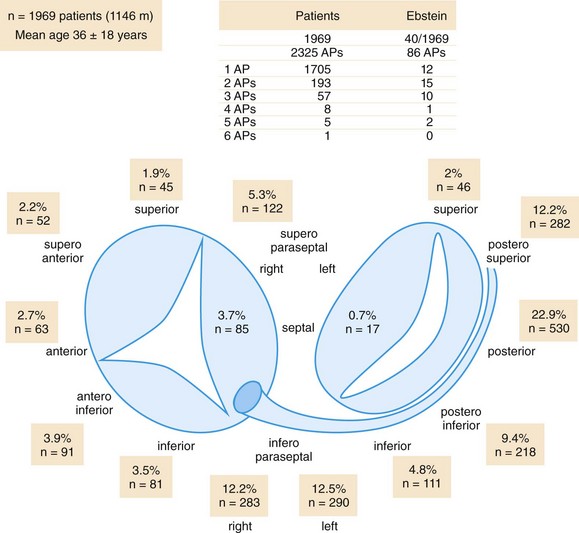
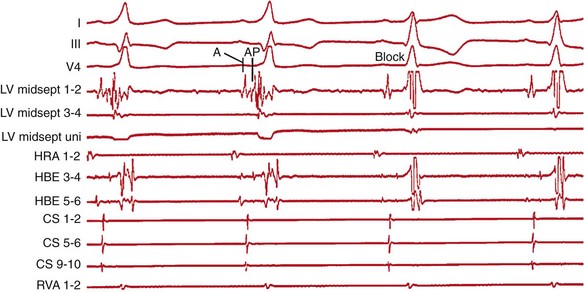
123 Current international guidelines firmly establish catheter ablation of supraventricular tachycardia as first-line therapy in patients with recurrent episodes.1 Historically, lessons from surgical resection of arrhythmic tissue paved the way for percutaneous catheter ablation. Currently, catheter ablation is typically performed as a combined procedure following a diagnostic electrophysiology study. This chapter will discuss the role of catheter ablation for the treatment of paroxysmal and persistent regular supraventricular arrhythmias, whereas ablation of atrial fibrillation is covered elsewhere. The ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias list catheter ablation as a class I indication in patients with recurrent atrioventricular nodal reentrant tachycardia (AVNRT).1 Catheter ablation is the preferred choice by most patients, preventing recurrent symptoms and the need for drug therapy. Catheter ablation of AVNRT is based on the concept of dual atrioventricular nodal (AVN) pathways with distinct anatomical and functional entities. Koch’s triangle describes the anatomical region incorporating the slow and fast AVN pathways. The His-bundle marks the apex of Koch’s triangle, whereas the fast AVN pathway borders just posteriorly, extending into the compact AVN. The tendon of Todaro forms a bandlike structure extending posteriorly and inferiorly from the AVN to the Eustachian ridge marking the posterior border of Koch’s triangle, whereas the ostium of the coronary sinus marks its base and the tricuspid valve is its anterior boundary. The slow pathway extends between the CS ostium and the tricuspid valve annulus toward the compact AVN and His bundle. It has been demonstrated that patients with AVNRT have a larger coronary sinus ostium than do control patients, a fact that needs to be appreciated when positioning the ablation catheter in the region of the slow pathway.2 In the pioneering days of radiofrequency (RF) ablation, the fast pathway was targeted for ablation, resulting in a prolonged PR interval during sinus rhythm and a high rate of complete AV block because of close proximity to the compact AVN.3 Currently, the standard approach is to target the slow pathway for ablation. Two techniques for successful slow AVN pathway ablation, slow pathway potential mapping, and an anatomical approach, have been described. Using a detailed mapping approach, Jackman et al.4 recorded large, sharp, slow pathway potentials along the inferior septum between the tricuspid annulus and the coronary sinus ostium that, if targeted during catheter ablation, resulted in modification or ablation of the slow pathway with a high rate of success.4 Haissaguerre at al.5 reported a high success rate following catheter ablation of typical slow/fast AVNRT targeting discrete low-frequency, low-amplitude potentials posteroinferior to the AVN and anterior to the coronary sinus ostium. Although the electrophysiological characterization of slow pathway potentials has been variable, targeting these potentials during ablation has proven highly effective. Alternatively, an anatomical approach to slow pathway ablation can be used. For this purpose, Koch’s triangle is divided into three sections as seen during fluoroscopic imaging. The superior third harbors the AVN and fast pathway and correlates fluoroscopically with the position of the His bundle recording catheter. The middle third extends inferiorly to the roof of the coronary sinus. The inferior third incorporates the coronary sinus ostium and is inferred fluoroscopically by the catheter positioned in the proximal coronary sinus. The inferior third of Koch’s triangle represents the typical site for slow pathway ablation. At this location, the ablation catheter will record a large ventricular signal and a single or multicomponent atrial potential with an atrial-to-ventricular range of 0.1 to 0.5. A conventional, nonirrigated, 4-mm tip ablation catheter in temperature mode is generally used with a target temperature of 55°C and an initial power of 20 W that is gradually titrated to 30 to 40 W if junctional ectopy ensues. If initial attempts are unsuccessful, the catheter is gradually advanced superiorly until energy application results in successful slow pathway ablation. It is important to note that ablation at a more superior position can increase the risk of inadvertent AV block because of closer proximity to the AVN. Rarely, if repeated attempts at common right atrial sites prove unsuccessful, RF application at the inferior left atrial septum is required for successful slow pathway ablation.6 Comparing the technique of detailed slow pathway potential mapping to an anatomical approach, a prospective randomized study found both methods to be comparable in efficacy, procedure duration, fluoroscopy time, and mean number of RF applications.1 Frequently, both techniques are combined scanning the inferior septum in the proximity of the coronary sinus ostium for a multicomponent atrial potential and a larger ventricular signal. As an alternative energy source, the use of cryoenergy for ablation of AVNRT was first reported by Skanes et al.7 The application of cryoenergy allows for testing of the functional impact of each freeze application at specific sites (cryomapping) before delivery of a permanent lesion. Ice formation at the catheter tip will prevent catheter dislodgment, and cryoablation is less painful to the patient.8 Cryoenergy application at the site of the slow pathway will not induce junctional rhythm and cannot be used to guide lesion delivery.7 During successful RF ablation of the slow pathway, an accelerated, irregular junctional rhythm is commonly seen. Albeit sensitive, development of a junctional rhythm is a nonspecific finding also noted in 65% of ineffective RF applications.9 Rarely, successful slow pathway ablation can be achieved in the absence of a junctional rhythm.10 Complete elimination of slow pathway conduction serves as an optimal endpoint for ablation. However, modulation of the slow pathway (i.e., presence of residual slow pathway conduction limited to single echo beats without inducibility of tachycardia after isoproterenol infusion) is an acceptable endpoint if AVNRT was inducible before ablation. In this scenario, slow pathway modulation demonstrates comparable long-term success rates to complete elimination of slow pathway conduction.11 In patients with baseline prolongation of the PR interval and AVNRT, the procedural endpoint should aim at modulating slow pathway conduction, since complete slow pathway ablation led to a higher rate of late complete AV block.12 By contrast, atrioventricular conduction can improve once slow pathway conduction has been eliminated, likely owing to reversal of electrotonic inhibition by the slow pathway imposed on the fast pathway.13 In approximately 10% of patients, AVNRT remains noninducible during the electrophysiology study despite the use of intravenous isoproterenol.14 Most commonly, this is due to a block in the fast pathway. If AVNRT cannot be induced in the presence of dual AVN physiology and the documented supraventricular tachycardia suggests AVNRT, slow pathway ablation should be performed, demonstrating excellent long-term results.1,15 The risk of atrioventricular block during ablation of the slow pathway has been estimated to be less than 1% in experienced centers.4,5 Unstable catheter tip-to-tissue contact owing to deep inspiration or anatomical features, such as a large coronary sinus ostium or a narrow Koch’s triangle, are often overcome by intensifying sedation and the use of a long sheath to facilitate catheter stability. Anterograde atrioventricular conduction is carefully monitored during RF application, and energy delivery is aborted if prolongation of the atrioventricular interval is noted. Induction of a fast junctional rhythm with a cycle length less than 350 ms was predictive for the development of atrioventricular conduction block.3 The ventriculoatrial interval should be monitored continuously, and stable catheter position should be verified to minimize the risk of AV block during energy delivery. Prolongation or block of ventriculoatrial conduction suggests imminent high-grade anterograde atrioventricular block, and immediate termination of RF delivery is warranted.3 Alternatively, ablation can be performed during atrial pacing at a rate sufficient to override the junctional rhythm while closely monitoring anterograde atrioventricular conduction. Radiofrequency ablation of the slow pathway has proved itself highly effective, with a reported acute success rate of 97% to 100%.1,4,5 During long-term follow-up 1.3% to 6.9% of patients develop recurrent AVNRT.1,16 Acute success rates for cryoablation are similar to that of RF ablation.17 However, in a prospective, randomized, multicenter study enrolling 509 patients, long-term results demonstrated a significantly higher rate of recurrence for patients undergoing cryoablation compared with RF ablation (9.4% vs. 4.4%). In light of these findings, RF ablation should be the first choice for patients undergoing ablation of the slow pathway. Catheter ablation is the mainstay of treatment for patients with AVRT, demonstrating excellent long-term outcome and a low rate of complications.18,19 Although low in prevalence, rapid anterograde conduction over an accessory pathway in the setting of atrial fibrillation can result in ventricular fibrillation and sudden cardiac death. The most recent ACC/AHA/ESC guidelines give catheter ablation a class I indication in patients with symptomatic AVRT because of a manifest (Wolff-Parkinson-White [WPW] syndrome) accessory pathway.1 AVRT in the presence of a concealed accessory pathway is a class IIa indication, unless AVRT is poorly tolerated. In the latter case, catheter ablation becomes a class I recommendation.1 Accessory pathways are commonly located along the tricuspid or mitral annulus or within the subepicardial pyramidal space in the inferoseptal region (Figure 123-1), forming connections between atrial and ventricular tissue. Rarely, an accessory pathway spans from the atrium (atriofascicular), AV node (nodofascicular or nodoventricular), or His bundle (His-fascicular) to a more distal branch of the His-Purkinje system or the ventricular musculature. The 12-lead surface electrocardiogram (ECG) will aid in pathway localization and preparation for the best ablation strategy. Arruda et al.20 developed an algorithm for accessory pathway localization correlating the surface 12-lead ECG pattern with the successful ablation site in 135 patients with manifest anterogradely conducting pathways.20 In a prospective evaluation of 121 patients, the authors could demonstrate that the vector of the initial portion of the surface delta wave in leads I, II, aVF, and V1, as well as the R-to-S ratio in lead III and V1 accurately predicted 1 of 10 sites around the atrioventricular annuli or subepicardial region with a sensitivity of 90% and a specificity of 99%.20 Figure 123-1 Distribution of accessory pathway location. Schematic of tricuspid and mitral atrioventricular (AV) rings, in a left anterior oblique projection, showing the locations of accessory pathways from 1969 consecutive patients. Anatomically correct terminology is used.25 The table shows the number of accessory pathways per patient in those with and without Ebstein anomaly. (From Ernst S, Ouyang F, Antz M, et al: Catheter ablation of atrioventricular reentry. In Zipes DP, Jalife J, editors: Cardiac Electrophysiology from Cell to Bedside, ed 4, Philadelphia, 2004, WB Saunders, pp 1078–1086.) Following successful catheter ablation, the 12-lead surface ECG commonly demonstrates repolarization changes in the direction of the delta wave. These alterations in repolarization are caused by cardiac memory and require no further treatment resolving spontaneously over time.21 Multiple accessory pathways can occur in up to 13% (see Figure 123-1) of patients with the WPW syndrome and its prevalence is as high as 52% in patients with Ebstein anomaly.22 The presence of additional accessory pathways may be evident only after ablation of the first, or it might be suggested during intracardiac mapping by variations in the activation sequence because of simultaneous or alternating conduction across multiple pathways. Accurate localization of an accessory pathway will require careful intracardiac mapping during the electrophysiology study. Ventricular myocardium is relatively thick at the atrioventricular groove, resulting in an atrial-to-ventricular-signal amplitude ratio less than 1, whereas a position at the atrial insertion site has a ratio of approximately 1 and a position at the ventricular insertion site a ratio of 1/2 to 1/6. Catheter stability is verified by less than 20% variation in the atrial potential amplitude.23 The most reliable mapping criterion is the recording of an accessory pathway potential. In manifest pathways, the distal bipolar recording at the successful ablation site will demonstrate a discrete, low-amplitude accessory pathway potential between the local atrial and ventricular electrogram that precedes the onset of the delta wave on the surface ECG (Figure 123-2). Generally, pacing maneuvers at atrial and ventricular sites are used to dissociate the pathway potential from the atrial and ventricular electrograms. Importantly, the number of RF energy applications necessary for successful pathway ablation is lower if an accessory pathway potential can be identified.3 Figure 123-2 Radiofrequency ablation of a right midseptal (paraseptal) accessory pathway. The top tracings show surface electrocardiograph leads I, III, and V4 with prominent delta wave in the first two beats, followed by disappearance of preexcitation in beats 3 and 4 once the accessory pathway is blocked (Block). Intracardiac tracings recorded from the ablation catheter (LV midsept 1-2, 3-4, and LV midsept uni) positioned at the insertion site of the midseptal (paraseptal) accessory pathway. The distal bipolar electrode (LV midsept 1-2) records an atrial signal (A) followed by an accessory pathway potential (AP) and a ventricular signal in beats 1 and 2. Note the prolongation of the local atrial-to-ventricular interval once conduction block in the accessory pathway occurs (beats 3 and 4). The unipolar electrode (midsept uni) located at the accessory pathway insertion site records a QS complex, indicating activation away from the unipolar electrode. HRA 1-2, Recording from the high right atrium; HBE 3-4 and 5-6, His-bundle recordings; CS 1-2, 5-6, and 9-10, recording from the coronary sinus; RVA 1-2, recording from the right ventricular apex. In clinical practice, the earliest local ventricular or atrial activation will identify the accessory pathway insertion site. In anterogradely conducting accessory pathways, the timing of the preexcited local ventricular electrogram on the bipolar recording during sinus rhythm, atrial pacing, or antidromic AVRT in reference to the earliest onset of the delta wave on the surface ECG is used to localize the ventricular insertion site of the accessory pathway accurately.24 At this site, the unipolar electrogram demonstrates a QS pattern indicating spread of activation from the insertion of the accessory pathway toward local ventricular tissue (see Figure 123-2). In the presence of a left accessory pathway, access is gained through a transseptal or a retrograde transaortic approach. Lesh et al. compared a transseptal to a retrograde transaortic approach and concluded that both complement each other, resulting in nearly 100% success if used in combination.1 In most cases, the primary determinant of which approach to choose is one of operator preference. Transseptal access carries the risk of systemic air embolism and cardiac tamponade owing to perforation, but it avoids aortic valve injury or coronary artery dissection using a retrograde transaortic approach.1 Transseptal access is preferred in children and older patients or in the presence of aortic valve stenosis, mechanical aortic valve prosthesis, or significant arterial disease. Reported success rates for ablation of left free wall accessory pathways range from 95% to 97%, but success rates are lower for right free wall accessory pathways (90%).1,3 A multielectrode mapping catheter positioned around the tricuspid annulus will facilitate localization of a right free wall accessory pathway. Catheter stability is improved by the use of a long sheath placed via the femoral vein. Ebstein anomaly is associated with a higher incidence of right accessory pathways and multiple right-sided pathways are present in 52% of patients (see Figure 123-1).22 In the region of the “atrialized” right ventricle, the presence of extensive fractionated endocardial electrograms can confound proper identification of local preexcited potentials and discrete pathway potentials.22
Ablation of Supraventricular Tachyarrhythmias
Ablation of Atrioventricular Nodal Reentrant Tachycardia
Indications
General Principles
Slow Pathway Ablation
Endpoints for Slow Pathway Ablation
Risk of Atrioventricular Block
Outcome
Ablation of Atrioventricular Reentrant Tachycardia
Indications
Accessory Pathway Localization
Free Wall Accessory Pathways
Ablation of Supraventricular Tachyarrhythmias



