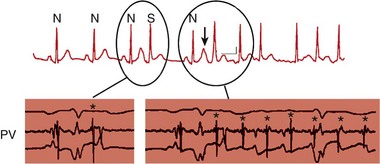
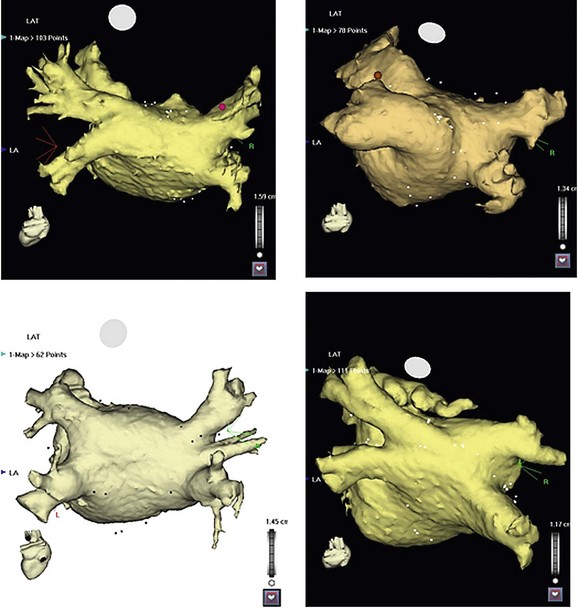
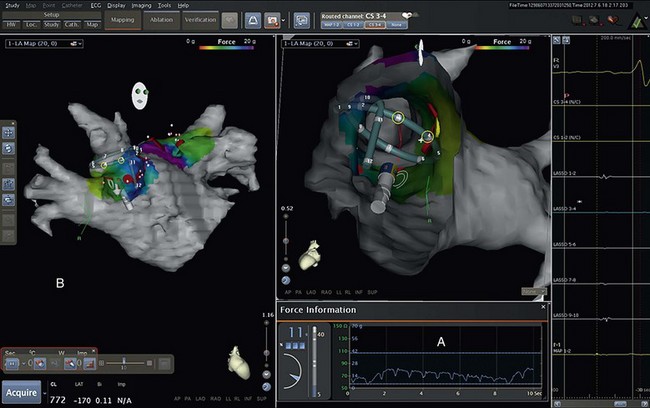
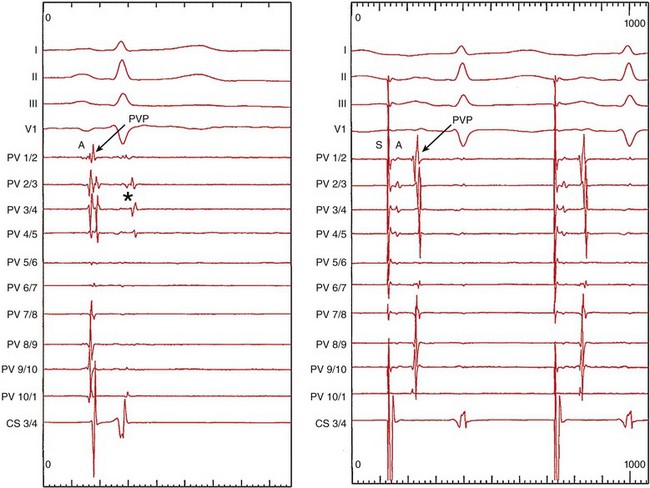
122 Since the initial description of pulmonary vein (PV) triggers,1 catheter ablation of atrial fibrillation (AF) has become a frequently performed ablation procedure. From a historical perspective, it is possible to trace the initial attempts at catheter ablation to the prior experience with surgical techniques. Cox et al.2 pioneered a surgical procedure based on the multiple wavelet reentry theory of Moe. Isolation of the posterior left atrium (LA) and segmentation of both atria using long linear obstacles aimed to reduce the mass of electrically contiguous atrial tissue below the critical threshold required for AF maintenance. To reduce complications and improve efficacy, the deployment of these linear obstacles was modified from Maze I to III. PV and posterior LA isolation has consistently been retained in each modification of the Maze procedure.3 The Maze procedure and its variants are the most widely performed curative surgical interventions for AF. Although the long-term success rate stands at 80%, and up to 99% with antiarrhythmic drugs, these surgical techniques remain complex and are associated with a significant increase in cardiopulmonary bypass time, morbidity, and mortality.3 Initial catheter ablation approaches were based on the experience from surgical methods of AF ablation.4 For safety reasons, linear lesions were initially limited to the right atrium; however, the success of this approach was less than 20% in patients with paroxysmal AF.5,6 In some series, early results were better, but significant attrition was observed at long periods of follow-up. Extension of linear lesions to the LA was demonstrated to be much more effective, even in persistent AF, particularly in the initial report of Swartz and Silvers.7 However, most published series reported significant complications as well as long procedural times.4 The subsequent demonstration of discrete triggers initiating AF, most often in or near the PVs, but also near other thoracic veins and atrial structures, prompted a shift toward a “focal” ablation strategy.1 The focal approach comprised mapping the earliest activity responsible for AF initiation followed by discrete ablation. Figure 122-1 demonstrates the characteristic features of AF triggered by PV focus. However, the limiting factors of this approach were rapidly identified. First, this approach supposes AF initiation at the time of the EP study and this arrhythmia are notoriously capricious in terms of spontaneous initiation. In addition to limited mapping opportunities, the elimination of one focus in a particular PV did not prevent another focus from the same vein, from its ostium, or from elsewhere firing at a later time, resulting in a high rate of AF recurrence. Provocative maneuvers were used to improve foci identification and ablation, but they frequently resulted in AF that required electrical cardioversion. Figure 122-1 Characteristic features of atrial fibrillation (AF) triggered by a pulmonary vein (PV) focus. The top panel demonstrates “P on T” monomorphic atrial ectopy on the surface electrocardiogram, with the second ectopic beat initiating AF (arrow). The bottom panel is the contemporaneous intracardiac recordings, with the middle tracing taken from the distal bipole of a quadripolar mapping catheter placed at the PV ostium. During ectopy (asterisk), the pulmonary vein potential is seen preceding the far-field atrial signal, whereas during AF, repetitive PV firing (asterisk) is demonstrated. The development of circular mapping catheters and the limited success and long procedural durations of the focal ablation technique paved the way for circumferential isolation of all PVs (and sometimes the superior vena cava), which would eliminate the electrical breakthroughs at the venous ostia.8 This can be considered a pragmatic approach to AF ablation because it assumes that all PVs can potentially harbor AF triggers without necessarily demonstrating PV arrhythmogenicity. Circumferential, en bloc isolation of PVs is now commonplace as a result of randomized studies, which suggest that this approach may be superior to segmental PV isolation, notably in persistent AF.9–11 PV isolation has been recommended at the level of the antrum to avoid PV stenosis and to encompass a greater amount of the arrhythmogenic substrate. During the past decade, ablation techniques, technology, and operator expertise have steadily improved. Therefore, catheter ablation has been progressively offered to a greater proportion of the global AF population, and this can be expected to continue. Patients must be assessed before catheter ablation, with attention to the management of comorbidities including hypertension, sleep apnea, obesity, cardiac ischemia, and heart failure. The rationale for catheter ablation of AF is to reduce symptoms and improve the quality of life. Catheter ablation of AF has not yet been demonstrated to reduce AF mortality and there is no randomized data to support a reduction in stroke risk. Current consensus guidelines recommend that ablation can be considered in symptomatic patients who have failed treatment with at least one antiarrhythmic drug or those who present with significant consequences of the arrhythmia such as tachycardiomyopathy.12 There are randomized data to support the superiority of catheter ablation over antiarrhythmic drugs in symptomatic patients with drug-refractory paroxysmal AF and a relatively normal left atrial size.13,14 Catheter ablation is not currently considered as first-line treatment for AF.12 Characterization of AF usually determines the overall ablation strategy because a greater degree of atrial remodeling generally accompanies the progression from paroxysmal to persistent and finally longstanding persistent AF. According to the guidelines, catheter ablation targeting the PVs is the cornerstone of most AF ablation strategies.12 In patients with persistent AF, additional ablation targeting atrial substrate is recommended to achieve acceptable clinical results. An ideal ablation strategy in this group of patients has not yet been determined. Transthoracic echocardiography should be performed within 6 to 12 months of ablation to evaluate the left atrial size (preferably by volumetric analysis) and to exclude significant valvular pathology or cardiomyopathic process. Preprocedure transesophageal echocardiography (TEE) can be used to screen for LA thrombus and to define the anatomy of the interatrial septum, including the presence of a patent foramen ovale. TEE may detect LA thrombus in patients who are taking therapeutic anticoagulants, and the predictors include a high CHADS2 score, LA size, and persistent AF.15 Although TEE is recommended in all persistent AF cases, this practice is variable in paroxysmal AF. Preablation cardiac computed tomography (CT) scanning with digital segmentation and three-dimensional reconstruction of the left atrial and pulmonary anatomy is an important aspect of ablation planning in most centers. High-quality CT images, obtained during sinus rhythm or AF, elegantly demonstrate the highly variable anatomy of the PV to the LA junction.16 The DICOM3 data obtained from the CT scan can be directly imported into three-dimensional mapping systems, or the data can be presented as an overlaid and linked image by the fluoroscopic imaging system to assist the operator during the procedure. The widespread use of CT imaging has largely obviated the need for intraprocedural pulmonary venography. CT imaging provides valuable information concerning the highly variable anatomy (Figure 122-2). The veins may demonstrate early branching or have a relatively long common trunk before the first branch.16 Early branching veins are more frequently encountered on the right side and must be ablated with caution to avoid PV stenosis. Amalgamation of both left-sided veins forming a common trunk is seen in approximately 30% of cases, whereas a third right-sided vein, the right middle PV, is seen in 18% to 29% of cases.12 Rarer variants may be seen, including roof veins.17 PV pseudostenosis is most commonly seen at the left inferior PV and is caused by the external compression by the descending aorta.18 The anatomy and location of the left atrial appendage is variable and the ostium may be located high or low relative to the mitral annulus.19 The ridge of tissue between the left-sided veins and left atrial appendage (LAA) is variable in its location, extent, and thickness.19 The variable location and presence of a fat pad between the esophagus and left atrium/coronary sinus is relevant for risk management because of the risk of thermal injury to the esophagus.20 Figure 122-2 Highly variable pulmonary vein (PV) anatomy. Three-dimensional reconstruction from 64-slice computed tomographic PV angiography demonstrates the highly variable anatomy of the PV–left atrial junction. The upper panels show a left common pulmonary trunk, and the lower panels demonstrate the right middle vein variant. Different PV caliber and branching patterns can also be appreciated from these images. Cardiac magnetic resonance imaging (MRI) with delayed enhancement has been described as a technique to visualize and quantify the amount of preexisting atrial fibrosis.21 Extensive studies from a single center have demonstrated that the quantum of preexisting LA fibrosis can predict the outcomes of catheter ablation of AF with a significantly lower likelihood of success as the area of delayed enhancement increases.22 This type of imaging also provides important insights into the atrial substrate, including the anchoring of critical areas for complex fractionated atrial electrograms (CFAEs) at border zones of preexisting scar tissue.23 The use of stronger magnets with improving image resolution, as well as the absence of ionizing radiation and the additional information provided by MR, makes it an imaging modality of higher preference than CT. A variety of three-dimensional navigation tools, such as Ensite NavX (Endocardial Solution, St. Paul, Minnesota) and CARTO (Biosense-Webster, Diamond Bar, California), which can integrate images from cardiac CT or MRI, may be used to assist with the procedure (Figure 122-3). Figure 122-3 Three-dimensional mapping with image fusion and demonstration of real-time contact force. This figure is a screen capture from an atrial fibrillation ablation procedure using the Carto 3 system (Biosense-Webster, Diamond Bar, California) with a SmartTouch contact force–sensing catheter (Biosense-Webster). The cardiac anatomy is provided by a preprocedure 3-T magnetic resonance imaging scan (with gadolinium contrast) that has been segmented and rendered by the mapping system. The variable 10-pole circumferential mapping catheter (variable Lasso, Biosense-Webster) is engaged within the right superior pulmonary vein, and the ablation catheter is positioned at the carina. Note the real-time contact force window (A) and the arrow indicating the force vector and amplitude (B). The color scale indicates the amount of force, in grams, delivered to each part of the venous ostium. The approach to anticoagulation before AF ablation varies. The safest approach is to determine anticoagulation requirements according to standard criteria and to manage the patient in a similar way to those considered for cardioversion. Thus, patients with a high CHADS2 or CHA2DS2-VASc score, hypertrophic cardiomyopathy, or valvular heart disease should be fully anticoagulated. Patients with an episode of AF lasting longer than 48 hours should also be fully anticoagulated. A majority of operators advocate systematic anticoagulation for all patients for one month before AF ablation.12 Preablation anticoagulation bridging may be performed using low-molecular-weight heparin or, alternatively, the procedure may be undertaken with the patient fully anticoagulated. The role of newer oral anticoagulant agents is uncertain because a higher stroke risk has been reported in a multicenter study24 and more data are required to assess the safety of these agents in association with AF ablation procedures. Pulmonary vein isolation can be performed using a wide variety of tools and techniques, with a universal end point of achieving electrical isolation between the PV muscular sleeves and the left atrium. Mapping of PVs is facilitated by the use of multielectrode circular mapping catheters.8 The recorded electrograms representing electrical activation of PV sleeves are called pulmonary vein potentials (PVPs). Electrical activation of the PVs is not homogeneous. The pulmonary venous activation from the left atrium often begins at one point, which can be considered to be one input or one connection. The electrograms recorded by the circumferential mapping catheter are a fusion of far-field and near-field signals. Closer electrode spacing reduces the amplitude of far-field signals; however, an understanding of the potential sources of far-field signals is critical to avoid misinterpretation and unnecessary ablation. During mapping of the left-sided veins, the left atrial appendage is the dominant source of far-field signal, which naturally is limited to the anterior half of the veins.25 Pacing the distal coronary sinus or directly pacing the left atrial appendage alters the activation sequence and is a practical and effective way to separate the far-field signals from the PVP (Figure 122-4). Posterior left atrial far-field signal is sometimes seen in the posterior half of the left-sided veins. It is best appreciated by directly pacing the posterior left atrium in close proximity to the venous ostia. Far-field signals are anticipated using this maneuver while PVP are delayed. In the right-sided veins, lower-amplitude right atrial far-field signal is seen on the anterior aspect. This signal corresponds to the onset of the surface electrocardiogram P wave and can be confirmed by directly pacing the right atrium. Less commonly, LA far-field signal can be seen at the posterior half of the right-sided veins. Pacing the posterior LA near the PV ostia can separate them from near-field PVPs. As ablation lesions are delivered circumferentially around the veins, the inputs are destroyed. Therefore, the PVP activation delays and its sequence changes. Electrical connections may exist between ipsilateral PVs and are more commonly seen on the left side.26 The PVs exhibit remarkable electrophysiological properties, including concealed firing and arrhythmia confined within the vein after isolation.27 The PV may support repetitive firing with cycle lengths as short as 110 ms, because these cells exhibit the shortest refractory period of cardiac tissue.28 Figure 122-4 Use of coronary sinus (CS) pacing during mapping of left-sided veins. This left superior pulmonary vein (PV) tracing during sinus rhythm demonstrates concealed PV ectopy (asterisk) synchronous with the QRS on the surface electrocardiogram, which disappears during CS pacing (right panel). During CS pacing (right panel), atrial far-field signals (A) are anticipated and the PVPs are more easily seen. In early experience, attempts were made to identify and isolate only arrhythmogenic PVs.29 Arrhythmogenic PVs may demonstrate frequent monomorphic atrial ectopic beats in a “P on T” pattern.1 Vector analysis algorithms and QRST subtraction software enable identification of the source of these ectopic beats.30 Limiting ablation to arrhythmogenic veins carries a higher rate of arrhythmia recurrence, and currently all PVs are routinely targeted and, in some centers, the superior vena cava (SVC) is also targeted for isolation. In the authors’ experience, the SVC is an uncommon source of AF (<1%), and AF originating from the inferior vena cava is more rare.31 The left-sided SVC has been demonstrated to be arrhythmogenic, and isolation of this structure often requires energy delivery from within the left SVC, as well as the left atrium.32 Pulmonary vein isolation can be performed during sinus rhythm, during paced rhythm (e.g., coronary sinus pacing to separate LAA far-field) or during ongoing AF. PV potentials can be identified during ongoing AF; however, it is more difficult to assess far-field signals because pacing cannot be used. The initial PV activity during ongoing AF is rapid and usually disorganized. As ablation is performed around the PV antrum, the PVPs become organized as more breakthroughs are destroyed.33 Often, high-degree block is seen immediately before isolation.33 PV isolation should be confirmed in sinus rhythm using pacing techniques to define far-field signal. The main cause of arrhythmia recurrence after PV isolation is recovery of PV conduction.12 This is the main issue of ablation to address. The insufficient development of ablation lesions may be caused by low tissue-contact force, inadequate energy/power delivery, or acute development of atrial edema. The durability of PV isolation is difficult to predict during the index procedure. A minimum of 20 minutes of observation after PV isolation is stipulated by the HRS/EHRA/ESC guidelines12 to enable assessment of acute conduction recovery. However, recovery at up to 1 hour of observation has been described.34 Adenosine testing can be used to assess PV isolation.35 Adenosine transiently hyperpolarizes the resting membrane potential of ablated PVs and restores excitability (so-called dormant conduction or reconduction) in 50% of isolated PVs.36 In nonrandomized series, adenosine testing after PV isolation with additional ablation targeting dormant conduction has reduced repeat procedure rates by approximately 50%.37 Preliminary results suggest that contact force technology will also improve the durability of PV isolation as better quality lesions can be delivered.38 HRS/EHRA/ESC guidelines recommend that atrial substrate ablation be considered in patients with persistent AF.12 Unfortunately, these patients often have complex and too-chaotic atrial electrograms to allow discrete analysis of the local activation cycle length, morphology, or activation. Distinguishing passive from active sites harboring critical atrial drivers remains the most challenging part of AF mapping. Different strategies aim to modify atrial substrate. Electrogram-based ablation targets complex fractionated atrial electrograms,39 high-frequency atrial activity,40 and discrete sources that activate the atria centrifugally.41 Linear ablation techniques modeled after the surgical maze procedure attempt to extinguish wandering atrial wavelets (or rotors) and macroreentrant loops. Although the autonomic nervous system is not specifically targeted using these strategies, the ganglionic plexi are often ablated along the trajectory of ablation lesions. Finally, new reports of direct mapping of sources including foci and rotating waves provide an insight into the mechanisms underlying human AF.42,43 Fractionated atrial electrograms were first described as continuous activity or electrograms with fibrillatory interval of less than 100 ms and were distributed irregularly across the right and left atria.40 CFAEs were subsequently described by Nademanee et al.39 as low-voltage electrograms (0.05 to 0.25 mV), with a cycle length shorter than 120 ms, displaying two or more deflections, or having a continuous deflection from baseline. In addition, regional differences in electrogram characteristics can often be observed: The septum or structures annexed to the LA such as the coronary sinus (CS) and LAA often display the most complex fractionation and rapid atrial activity.44 CFAEs represent areas of slow conduction, zones of colliding wave fronts, or pivot points between different wavelets or ganglionic plexi, and as such do not indicate active participation in the AF process.39,45 The initial description and ablation results encouraged many groups to target CFAEs as either a sole strategy or in combination with PV isolation and linear ablation. The characterization of CFAEs as either active or passive is challenging to determine. The passive CFAEs can result from a remote driver accelerating the local tissue beyond its refractory period or may reflect asynchronous activation of local muscle bundles during periods of short cycle length. Shortening of the AF cycle length (by at least 10 ms) has been shown to precede complex fractionation in 91% and 88% of the regions sampled in the atria and coronary sinus, respectively, implying that fractionation is a consequence of higher-frequency activity.46 These passive CFAEs displayed nearly simultaneous activation covering only a limited part of AF cycle length, whereas active CFAEs display repetitive bursts and activities spanning the majority of AF cycle length, potentially indicating a rotating wave.46 The relationship among CFAEs, high-frequency electrograms, and the autonomic nervous system has been a subject of interest among investigators. Experiments in a sheep model of AF have shown that atrial myocardium exhibiting most fractionation occurred in areas of high-frequency activity.45 Directly stimulating the intrinsic cardiac ganglionic plexi in dogs results in the occurrence and sustainability of CFAEs; ablation of the ganglionic plexi resulted in significant attenuation or elimination of CFAEs.47 CFAEs have also been shown within sites with dominant high-frequency electrograms using spectral analysis.45 All of these animal studies advocate the targeting of CFAEs during catheter ablation. Commercially available three-dimensional mapping systems incorporate CFAE mapping algorithms and some have been evaluated in large randomized trials that did not show superiority over visual estimation by the operator. In two studies, electrogram characteristics were investigated to determine their impact on AF cycle length and AF termination. The best predictor associated with significant prolongation of the AF cycle length was atrial sites displaying continuous electrical activity (>70% of fibrillatory interval), with positive and negative predictive values of 52% and 67%, respectively.48 Other variables such as fractionation index (number of deflections of fractionated activity in a 4-second window), electrogram voltage, or local mean cycle length were not significant predictors. Cycle length slowing during CFAE ablation is not simply caused by atrial debulking, because ablating certain types of CFAEs had a greater effect, suggesting that these areas are more important for maintaining AF.49,50 The atrial fibrillatory cycle length (AFCL) is generally considered a surrogate for local tissue refractoriness. Earlier studies have shown that AFCL accurately tracks the shortening in local refractory periods seen with prolonged AF duration, or the lengthening in local refractory periods during antiarrhythmic drug therapy. The gradual prolongation in AFCL before AF termination during catheter ablation supports the use of AFCL to monitor substrate modification during AF ablation.44
Ablation for Atrial Fibrillation
Historical Context
Indications and Preablation Assessment
Pulmonary Vein Isolation
Atrial Substrate Ablation
Electrogram-Guided Ablation of Atrial Substrate
Monitoring the Progress of Ablation Using Atrial Fibrillatory Cycle Length
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Ablation for Atrial Fibrillation
