Chapter 130
Abdominal Aortic Aneurysms
Evaluation and Decision Making
Peter J.E. Holt, Matt M. Thompson
Based on a chapter in the seventh edition by Mark F. Fillinger
Background
Size Definitions
Aneurysms are defined as focal dilatations at least 50% larger than the expected normal arterial diameter.1 A practical working definition of an abdominal aortic aneurysm (AAA) is a transverse diameter of 3 cm or greater, and for a common iliac aneurysm, a transverse diameter greater than 1.8 cm, based on average values for normal individuals. The normal aortic diameter gradually decreases from the thorax (28 mm in men) to the infrarenal location (20 mm in men).2 At all levels, the normal aortic diameter is approximately 2 mm larger in men than in women and increases with age and increased body surface area.2 A large ultrasound screening study found that increasing age, male gender, black race, and increasing height, weight, body mass index, and body surface area were independently associated with increased infrarenal aortic diameter, but that the effects of all these variables were small.3 Rather than being termed atherosclerotic, AAAs are more accurately referred to as degenerative or nonspecific in etiology. Degenerative aneurysms account for more than 90% of all infrarenal AAAs.
Aneurysm Locations
Nearly all AAAs involve the infrarenal aorta, but about 5% to 15% of AAAs undergoing open surgical repair also involve the suprarenal aorta.4,5 By definition, because suprarenal AAAs extend above the renal arteries, they require re-implantation of at least one renal artery during AAA repair. The term juxtarenal is used to describe AAAs that do not involve the renal arteries, but because of proximity, require a clamp site above the renal arteries to complete the proximal aortic anastomosis in open repair or necessitate a fenestrated endograft in endovascular repair. Although up to 40% of AAAs also involve the iliac arteries,4,6 isolated iliac artery aneurysms are rare (<1% of aortoiliac aneurysm repairs).7 Isolated aneurysms of the suprarenal aorta are extremely rare, unless they have an associated thoracic or infrarenal component. Historically, concomitant thoracic aneurysms have been found in 12% of patients with AAAs.8 More recently, in a study of 1082 patients with a known AAA who had a computed tomography (CT) scan of the chest, a total of 253 patients (23%) were diagnosed with a thoracic aneurysm: 117 patients (10.8%) had a synchronous aneurysm and 136 (12.6%) had a metachronous thoracic aneurysm.9 Of all patients with known AAAs who were found to have a thoracic aneurysm, 61 of 253 (24%) underwent repair, had a rupture, or had a thoracic aortic aneurysm (TAA) >5.5 cm. Peripheral aneurysms of the femoral or popliteal artery are present in approximately 15% of patients with AAAs, with a strong male predominance for this association. Importantly, many of these peripheral aneurysms are not discernable on clinical examination, and therefore, a diagnosis must be actively sought.10,11
Epidemiology
Impact of Rupture
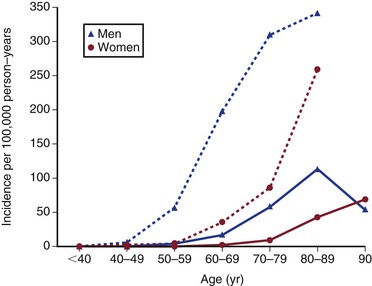
In the United States, ruptured AAAs are the 15th leading cause of death overall and the 10th leading cause of death in men older than 55 years, with the death rate increasing with age (Fig. 130-1).12 Overall, the age standardized death rate from ruptured AAAs in the population older 45 years old in the United States between 1999 and 2009 was 5.6 per 100,000 individuals, being significantly higher in males (9.0/100,000) and lower in females (3.2/100,000). In the most at risk groups, the rates are even higher, being 11.3 per 100,000 in whites, males, and non-Hispanics older than 55 years. The reason for these high death rates is that only half of patients with AAA rupture survive to reach the hospital, many of whom do not have a known diagnosis of AAA before aneurysm rupture. Historically, about a half are offered surgery, although this proportion might now be increasing, and the surgical mortality rate is approximately 50%, with 15% dying intraoperatively.13 The proportion of patients with ruptured AAAs who are offered surgery in individual hospitals appears to be hugely variable, and every attempt should be made to initiate treatment where possible and in accordance with the patient’s wishes.14
Figure 130-1 Crude death-rate per 100,000 population in the United States by age and gender from abdominal aortic aneurysms (ruptured and intact). (From the Centers for Disease Control and Prevention, National Center for Health Statistics: Compressed Mortality File 1999-2009. CDC WONDER Online Database, compiled from Compressed Mortality File 1999-2009 Series 20 No. 2O, 2012. Available at: http://wonder.cdc.gov/cmf-icd10.html. Accessed November 23, 2012.)
That said, there is mounting evidence from both the United States and the United Kingdom that not only is the prevalence of AAA falling, but the rate of mortality from aneurysm rupture is also decreasing15–17 (Figs. 130-2 and 130-3). This is likely due to a combination of factors, including better medical management of patients with cardiovascular disease, the advent of population aneurysm screening programs, a greater overall availability of cross-sectional imaging leading to earlier detection of AAAs, and finally, to a reduction in population smoking rates. The advent of endovascular aneurysm repair may also be playing a positive role on aneurysm population rupture rates and deaths. The treatment rate of patients with AAAs has substantially increased due to this minimally invasive technology being deliverable in patients who would be at high risk for traditional aneurysm repair, particularly in those older than 75 years of age. Despite these improvements, aneurysm rupture remains a common cause of death and morbidity, and an entity with a high associated mortality.
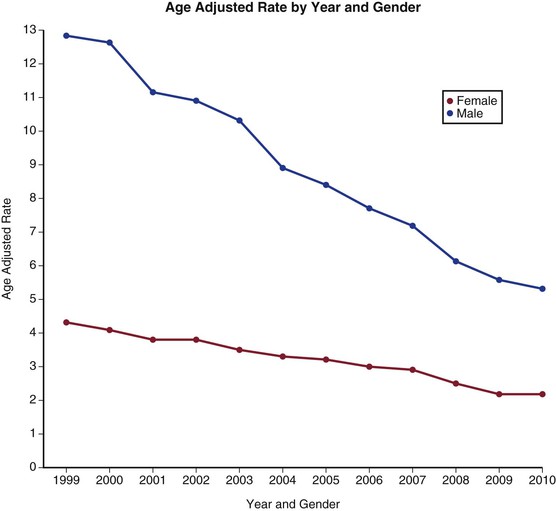
Figure 130-2 Age-adjusted death rate per 100,000 population by year and gender from ruptured abdominal aortic aneurysms in the United States from 1999 to 2009. (From the Centers for Disease Control and Prevention, National Center for Health Statistics: Compressed Mortality File 1999-2009. CDC WONDER Online Database, compiled from Compressed Mortality File 1999-2009 Series 20 No. 2O, 2012. Available at: http://wonder.cdc.gov/cmf-icd10.html. Accessed November 23, 2012.)
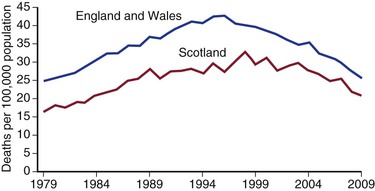
Figure 130-3 Deaths per 100,000 population in the United Kingdom from ruptured abdominal aortic aneurysm. (From Anjum A, et al: Is the incidence of abdominal aortic aneurysm declining in the 21st century? Mortality and hospital admissions for England & Wales and Scotland. Eur J Vasc Endovasc Surg 43:161-166, 2012.)
Incidence
AAAs primarily affect the population older than 50 years. AAAs are two to six times more common in men than in women, and are two to three times more common in white men than in black men.18–21 In men, AAAs begin to occur at approximately 50 years of age and reach a peak incidence at approximately 80 years of age.22–24 In women, AAA onset is delayed, beginning at approximately 60 years, with the incidence continuing to increase thereafter. Screening studies have provided information on both the prevalence and incidence of AAAs.
In the Huntingdon, United Kingdom, screening program for men older than 50 years, the incidence of new AAAs was 3.5 per 1000 person-years.25 The most recent data from the U.K. National Aneurysm Screening Programme showed that of the 107,051 men ages 65 years offered screening in 2011, only 1.5% had aortic diameters more than 3 cm.26 The difference between the two reports might reflect public health measures to reduce smoking and to improve cardiovascular health.
In the United States, a screening study of male veterans found new AAAs in 2.6% of patients 4 years after an initial normal aortic ultrasound study, for an incidence of 6.5 per 1000 person-years.27 Similarly, in the Huntingdon study, new AAAs were discovered in 2% of patients screened a second time at a mean of 5.5 years after an initial negative study. These data highlight the relevance of screening and potentially re-screening of the at-risk population to fully detect aneurysms within that population.
These figures are reflected in the rates of AAA-related death when reported by age and gender (Fig. 130-2).22–24 Overall, the age-adjusted incidence of both asymptomatic and ruptured AAAs, and of AAA-related death, is twofold to sixfold higher in men than in women. A significant increase in the incidence of asymptomatic AAAs has been noted in the past 2 decades,19,24,28 in part because of increased case detection as a result of more frequent use of ultrasonography and other imaging modalities. However, there is emerging evidence that the number of admissions for aneurysm repair is reaching a plateau (Fig. 130-4), perhaps due to the effects of public health measures aimed to reduce morbidity and mortality from cardiovascular diseases. These include the increased use of antihypertensive medications and statins, as well as smoking cessation programs and national antismoking campaigns.
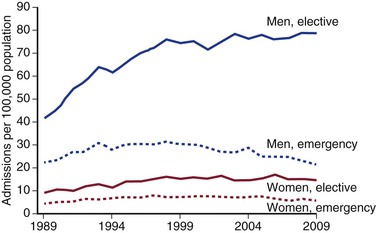
Figure 130-4 Admissions with aortic-related pathologies per 100,000 population in the United Kingdom by gender and mode of admission. (From Anjum A, et al: Is the incidence of abdominal aortic aneurysm declining in the 21st century? Mortality and hospital admissions for England & Wales and Scotland. Eur J Vasc Endovasc Surg 43:161-166, 2012.)
Incidence of Rupture
The reported incidence of ruptured AAA varies from 1 to 21 per 100,000 person-years.28 For patients older than 50 years, the incidence of AAA rupture is much higher because AAA increases dramatically with age (see Figs. 130-2 and 130-5).29 A population-based study of ruptured AAAs noted an incidence of 76 per 100,000 person-years for men and an incidence of 11 per 100,000 person-years for women older than 50 years, for a male/female ratio of 6.9 : 1.30 The median age at rupture was 76 years in men and 81 years in women. The median AAA size at rupture was 8 cm, but 4.5% of the ruptured AAAs were less than 5 cm in diameter (measured at autopsy or surgery). The overall mortality associated with rupture was 78%, and three quarters of these deaths occurred outside the hospital. It appears that the number of admissions with, and deaths from, aortic rupture are falling in more recent years following a period of sustained year-on-year growth. Other than better medical management of patients with small aneurysms, the reduction in smoking, and the advent of population AAA screening programs, it has been suggested that one reason for this decline is a more aggressive approach to AAA repair in more elderly patients, facilitated by the routine availability of endovascular aneurysm repair (EVAR).
Prevalence
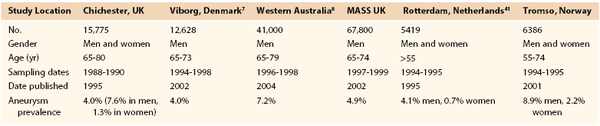
Prevalence estimates for asymptomatic AAAs (likelihood of having an AAA) are more accurate than incidence estimates (likelihood of an AAAs developing) now that large ultrasound screening surveys have been performed. Population ultrasound screening studies offer the best current evidence regarding the prevalence of AAAs (>3 cm). These studies have been conducted both as epidemiologic screening studies (such as Tromso and Rotterdam), and in a number of cases, as randomized controlled trials to assess the benefits of screening (Multicentre Aneurysm Screening Study [MASS], Western Australia, Viborg and Chichester). In a Veterans Affairs (VA) screening study of more than 73,000 patients 50 to 79 years old, the prevalence of AAAs ≥3 cm was 4.6% and that of AAAs ≥4 cm was 1.4%.31 The prevalence of AAAs reported varied according to both age and gender (Table 130-1). The highest prevalence of AAA ≥3.0 cm was 5.9% and was found in white male smokers between the ages of 50 and 79 years. These figures compare well with more recent estimates available from randomized trials of screening (Table 130-2).32
Table 130-1
Prevalence Estimates of AAA Based on Race, Sex, and Smoking History
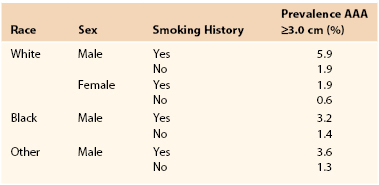
AAA, Abdominal aortic aneurysm.
Influence of Risk Factors
The prevalence of AAAs in a given population depends on the presence of risk factors associated with AAAs, including older age, male gender, white race, positive family history, smoking, hypertension, hypercholesterolemia, peripheral vascular occlusive disease, and coronary artery disease (CAD).33 Although these risk factors are associated with increased AAA prevalence, they may not be independent predictors and may be markers rather than causes of AAA disease. Of these risk factors, age, gender, and smoking have the largest impact on AAA prevalence.19,28,34 In the VA study, and similar epidemiologic studies, smoking was the risk factor most strongly associated with AAAs.31 The relative risk of having an AAA ≥4 cm (versus <3 cm) was fivefold higher in smokers than in nonsmokers, and the risk increased significantly with the number of years of smoking. The excess prevalence associated with smoking accounted for 75% of all AAAs that were ≥4 cm.31 Other important risk factors that increased AAA prevalence in this study were male gender (5.6-fold risk), age (1.7-fold risk per 7-year increase), white race (2-fold risk), and positive family history (2-fold risk); diabetes decreased the risk (0.5-fold risk). Less important independent risk factors for increased AAA prevalence were height, CAD, any atherosclerosis, high cholesterol level, and hypertension.
In a pooled study of more than 3 million individuals, the relative risk for aortic aneurysm–related events in current smokers was generally 3 to 6, as opposed to 1 to 2 for CAD or cerebrovascular disease and 5 to 12 for chronic obstructive pulmonary disease (COPD).35 Among smokers, AAA prevalence in one study was increased not only by the number of cigarettes smoked and increasing depth of inhalation, but also by elevated mean arterial or diastolic blood pressure.36 In another study of male smokers, AAA prevalence increased with increasing age, years of smoking, systolic and diastolic blood pressure, and cholesterol.37 Although there is less agreement that hypertension increases AAA prevalence, it does increase rupture risk in patients with established AAAs.33,36,38–42 Larger screening studies using multivariate analysis that included patients whose hypertension had been controlled with medications have found hypertension to be an independent predictor for the development of AAAs.31,39,42
More recently, genome-wide association studies have revealed a specific association between a genetic variant of low-density-lipoprotein receptor-related protein 1 and patients with AAA. This may provide a future target for the early detection and management of AAA.43
Familial Clustering
Familial clustering of patients with AAAs has been well described in the literature. The stated relationships are variable depending on the results of individual studies, but accepted estimates are that, of patients undergoing AAA repair, 15% to 25% have a first-degree relative with a clinically apparent AAA compared with only 2% to 3% of age-matched control patients without AAAs.44–51 Conversely, ultrasound screening of siblings of a patient with an AAA yielded AAAs ≥3 cm in 25% of the male and 7% of the female siblings older than 55 years.51 The likelihood that relatives will have an AAA increases if the patient with the AAA is a woman.52 It is estimated that first-degree relatives of a patient with an AAA have a 12-fold increased risk for aneurysm development themselves.48 Patients with familial aneurysms are on average 5 to 7 years younger and are more frequently women46,50 (Table 130-3).
Table 130-3
Independent Risk Factors for Detecting an Unknown 4-cm-Diameter or Larger Abdominal Aortic Aneurysm during Ultrasound Screening
| Risk Factor | Odds Ratio* | 95% CI |
| INCREASED RISK | ||
| Smoking history | 5.1 | 4.1-6.2 |
| Family history of AAA | 1.9 | 1.6-2.3 |
| Older age (per 7-yr interval) | 1.7 | 1.6-1.8 |
| Coronary artery disease | 1.5 | 1.4-1.7 |
| High cholesterol | 1.4 | 1.3-1.6 |
| COPD | 1.2 | 1.1-1.4 |
| Height (per 7-cm interval) | 1.2 | 1.1-1.3 |
| DECREASED RISK | ||
| Abdominal imaging within 5 yr | 0.8 | 0.7-0.9 |
| Deep venous thrombosis | 0.7 | 0.5-0.8 |
| Diabetes mellitus | 0.5 | 0.5-0.6 |
| Black race | 0.5 | 0.4-0.7 |
| Female gender | 0.2 | 0.1-0.5 |
* Odds ratio indicates relative risk in comparison to patients without that risk factor.
AAA, Abdominal aortic aneurysm; CI, confidence interval; COPD, chronic obstructive pulmonary disease.
From Lederle FA, et al: The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med 160:1425, 2000.
Clinical Findings and Diagnosis
Although AAAs are primarily a disease of the elderly, they can develop in patients younger than 50 years old. AAAs in younger patients are more often symptomatic, familial, and on average 1 cm larger at initial evaluation than in older patients.53 This finding may relate to fewer incidental abdominal imaging studies performed in younger patients, and a lower index of suspicion among clinicians, such that AAAs escape detection until they are larger or symptomatic. Younger patients tend to have more proximally located AAAs, with 46% being juxtarenal or higher as opposed to 18% at this level in older patients. Smoking is nearly universal in young patients with AAAs, whereas only 23% have a defined etiology, such as Marfan’s syndrome.53
Intact Abdominal Aortic Aneurysms
Most nonruptured AAAs are asymptomatic and discovered during abdominal imaging for an unrelated condition. Occasionally, patients may feel a “pulse” in their abdomen or palpate a pulsatile mass. Rarely, large AAAs cause symptoms from local compression, such as early satiety, nausea, or vomiting from duodenal compression; urinary symptoms secondary to hydronephrosis from ureteral compression; or venous thrombosis from iliocaval venous compression. Posterior erosion of AAAs into adjacent vertebrae can lead to back pain. Even without bony involvement, AAAs can cause chronic back pain or abdominal pain that is vague and ill defined. Acute ischemic symptoms can result from distal embolization of the thrombotic debris contained within an AAA. Ischemic symptoms appear to be more common with smaller AAAs, especially if the intraluminal thrombus is irregular or fissured.54 Acute thrombosis of an AAA occurs rarely but causes catastrophic ischemia. Atheroembolism is much more common than acute AAA thrombosis, but both combined occur in less than 2% to 5% of patients with AAAs.54 Nonetheless, an aortic aneurysm source for distal atheroemboli must always be considered, especially in patients without overt atherosclerotic occlusive disease. Such symptoms are nearly always an indication for AAA repair.
Ruptured Abdominal Aortic Aneurysms
Most AAAs that become symptomatic do so because of rupture or acute expansion, or the formation of penetrating aortic ulcers. In a review of stable but symptomatic patients with a suspected ruptured AAA, CT showed a ruptured aneurysm in 30%, a nonruptured AAA in 50%, and other pathology to explain the symptoms in 20% of patients.55 This is valuable information, because patients with symptomatic but nonruptured aneurysms (termed acutely expanding) have a substantially higher operative mortality rate than do those undergoing elective AAA repair (average of 23%).14,56,57 One large study reporting national results suggested that this relationship appeared to be maintained even in the setting of EVAR (Fig. 130-6).14 It is accepted, however, that the reported outcomes in this setting are varied, with some studies showing only a small difference in the mortality rates of EVAR for “urgent” AAAs compared with elective surgery. This higher mortality probably results from a combination of factors, including suboptimal preoperative evaluation, case planning, and management of comorbid disease, as well as potentially less experienced emergency surgical and anesthesia teams. Emergency repair is historically less likely to be endovascular, with the attendant differences in mortality rates. Because acute expansion is considered an immediate precursor of rupture, however, such patients must undergo expeditious AAA repair.
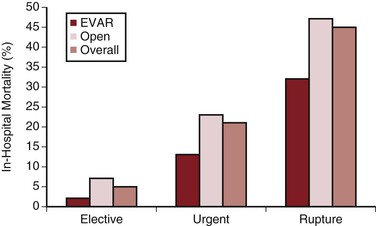
Figure 130-6 Comparative in-hospital mortality rates for endovascular aortic repair (EVAR) and open abdominal aortic aneurysm repair in the United Kingdom by mode of admission. (From Holt PJE, et al: Propensity scored analysis of outcomes after ruptured abdominal aortic aneurysm. Br J Surg 97:496-503, 2010.)
Physical Examination
Although most clinically significant AAAs are potentially palpable during routine physical examination, the sensitivity of this technique depends on AAA size, obesity of the patient, skill of the examiner, and focus of the examination.58 With physical examination alone, the diagnosis is made in 29% of AAAs 3 to 3.9 cm, 50% of AAAs 4 to 4.9 cm, and 75% of AAAs 5 cm or larger.59 Although a focused physical examination detected 50% of 3.5- to 6-cm diameter AAAs, they had all been missed on a recent, nonfocused examination.58 Conversely, AAAs may be falsely suspected in thin patients with a prominent but normal-sized aorta or in patients with a mass overlying the aorta that transmits a prominent pulse. Patients with hypertension, a wide pulse pressure, or a tortuous aorta can also have prominent aortic pulsation that may be mistaken for an AAA. The positive predictive value of physical examination for identifying AAAs greater than 3.5 cm in diameter is only 15%.60 The accuracy of physical examination in measuring the size of a known AAA is also poor, with the size usually being overestimated because of intervening intestine and abdominal wall tissue.
As a result of these factors, most AAAs are detected by incidental abdominal imaging studies performed for other reasons. In a review of 243 patients who underwent elective AAA repair, Chervu et al61 found that 38% were initially detected by physical examination, whereas 62% were detected by incidental radiologic studies, although 43% of them were palpable on subsequent examination. Of these clinically significant AAAs, 23% were not palpable even when the diagnosis was known, and in obese patients, two thirds were not palpable. These findings emphasize the fact that many significant AAAs are missed on physical examination and that ultrasound screening has a potential role in high-risk patients, as discussed later.
Imaging
Ultrasound
Several imaging modalities are available to confirm the diagnosis of AAA. Abdominal B-mode ultrasonography is the least expensive, least invasive, and most frequently used examination, particularly for initial confirmation of a suspected AAA and follow-up of small AAAs. Measurements of diameter with ultrasound have an interobserver variability of approximately 5 mm in 84% of studies and are more accurate in the anteroposterior than in the lateral dimension.62 Visualization of the suprarenal aorta and iliac arteries may be obscured by bowel gas or be difficult in obese patients. Ultrasonography cannot accurately determine the presence of rupture63 and often cannot accurately determine the upper extent of an AAA.64 When compared with CT, ultrasound typically underestimates the diameter of AAAs by 2 to 4 mm in the anteroposterior direction.62,65–67 In general, ultrasound is used to diagnose and monitor AAAs until the aneurysm approaches a size at which repair may be considered, whereupon more advanced imaging is used for preoperative evaluation.
Computed Tomographic Angiography
Computed tomographic angiography (CTA) is more expensive than ultrasound and involves exposure to radiation and intravenous contrast material, but it provides more accurate measurement of diameters, with 91% of studies showing less than 5-mm interobserver variability.61 Accuracy can be increased by using standardized techniques, electronic calipers, and magnification.65 Intraobserver variability under these conditions is within 2 mm in 90% of cases.65 Ultrasonography and CTA can overestimate AAA diameter if an oblique, rather than a perpendicular, section is obtained in a tortuous aneurysm. This results in an elliptic rather than a circular cross-section, and unless the aneurysm cross-section is truly asymmetric, the larger diameter of the ellipse overestimates the true AAA diameter. For this reason, CTA must be combined with three-dimensional reconstruction of the aorta with centerline of flow reconstructions and orthogonal views to accurately assess the aorta for endografting and to appreciate the morphologic challenges and features of a particular aneurysm. CTA is the most widely employed imaging modality for the immediate pre-operative assessment of patients with AAAs, both in the acute and elective setting. CTA has been successfully used in the acute setting both to confirm or exclude aortic rupture, and to plan endografting for the repair of AAA rupture.
CTA precisely defines the proximal and distal extent of an AAA, more accurately images the iliac arteries, and provides other important information for operative planning. This is particularly true with modern multidetector spiral CT with thin slices in the region of interest. Spiral CT allows not only accurate size measurements but also accurate definition of the relationship of an AAA to the visceral and renal arteries with CTA. Accordingly, CTA yields important information regarding the nature (open versus endovascular), extent, and complexity of surgery. Recent studies also indicate that incidental findings are present in the majority of CT scans, with 19% being clinically significant (e.g., tumor, horseshoe kidney, venous anomalies).68 For juxtarenal or suprarenal aneurysms, CTA can define the most appropriate location for aortic cross-clamping or feasibility for fenestrated or branched endografting. CTA with associated three-dimensional reconstruction has largely supplanted catheter angiography in the pretreatment assessment of AAA patients.69 For these reasons, CTA is the primary imaging modality in preoperative planning for AAA repair.
Rotational Angiography.
On-table rotational angiography has been used in some centers for the completion of imaging after EVAR. It has been suggested that the same technique can be employed to assess aneurysm morphology in the setting of AAA rupture. Pilot studies have compared the morphologic information gleaned from standard CT to that of rotational angiography with positive results. As well as providing confirmation of the diagnosis of aortic rupture in an operating theater setting, and therefore minimizing delay, the images can be used for case planning and endograft sizing.70,71 The major limitations appear to be a reduction in overall clarity of imaging compared with multidetector CT, difficulty in imaging the iliacs and access vessels due to the current limitations in the size of detector heads, and what appears to be a systematic undermeasuring of diameters compared with multi-detector CT.70–72 Clearly, this is an evolving technique, but one that might well find a role as operating theater imaging technology (software and hardware) improves and in specialist centers where EVAR predominates.
Magnetic Resonance Imaging.
The absence of exposure to ionizing radiation makes the use of magnetic resonance imaging (MRI) for AAA diagnosis and surveillance an attractive prospect. However, in many health care settings, the high cost and limited availability of MRI make this an impractical solution at the current time. Magnetic resonance angiography (MRA) is comparable to CTA in many respects with regard to accuracy of AAA measurement and evaluation, and it avoids radiation exposure. Despite the lack of ionizing radiation, MRA has not been the favored preoperative imaging technique, because compared with CTA, it visualizes calcified plaque poorly, typically has half the spatial resolution, is more expensive, is technically more difficult to standardize, and is less well tolerated by claustrophobic patients. Historically, MRA was used in patients with renal failure, but recent studies have shown the risk of potentially fatal nephrogenic sclerosing fibrosis in patients with renal insufficiency who receive gadolinium contrast.73,74 MRA with gadolinium is still useful when intravenous contrast administration is contraindicated because of allergic reaction, and flow-induced contrast (time-of-flight) can be used as an alternative. In centers with sufficient experience using this technique, MRA has been shown to be accurate in determining the presence of occlusive disease in intra-abdominal arteries. Improvements in the spatial resolution of spiral CTA, combined with its more rapid, less expensive technique, have largely relegated MRA to a secondary role in the evaluation of AAAs.
Screening
Because asymptomatic AAAs are often not discovered until they rupture, the potential benefit of ultrasound aneurysm screening programs is apparent. Targeted ultrasound screening has been shown to reduce both aneurysm-related mortality and all-cause mortality in screened individuals. Which individuals to target remains a topic of considerable debate, but it would seem sensible to focus effort on people most at risk (i.e., older males). Whether this is further limited to smokers or those with a family history of AAA is a point for discussion.
The importance of screening in the decision-making algorithm is predicated on the concept that vascular surgeons in modern vascular institutes aim to provide the best treatment to a population and to individuals. Therefore, the early detection of AAA moves patients out of the group of potential emergency admissions or aneurysm rupture into the elective cohort. This means that patients can have their comorbidities fully optimized and aortic morphology assessments made before surgery. This allows all treatment options to be considered, including custom-made devices, and the correct strategy planned for individual patients with commensurate improvements in outcome. These improvements can be demonstrated across screened communities and in individual patients. Screening programs also provide a platform for effective workforce planning as the approximate number of cases in any given community is known and the hospital in which they will be treated can be determined, avoiding emergency admissions with aortic rupture to less specialized units. This, in turn, has beneficial effects through volume-outcome relationships (see Prediction Algorithms for Endovascular Aneurysm Repair).
Prospective Trials
Although the individual screening studies are discussed in the following, the results of the key studies are summarized.75
Nonrandomized Trials
In the Gloucestershire Aneurysm Screening Programme, all men ages 65 to 73 years were offered ultrasound screening.76 No prescreening evaluation of potential fitness for AAA repair by a general practitioner was made. The attendance rate was 84%. AAAs more than 4 cm were detected in 2.2% of those scanned, and during subsequent follow-up, there was a 66% reduction in overall AAA-related mortality (including deaths resulting from elective repair and rupture), which was statistically significant. In the Huntingdon trial, general practitioners reviewed their lists of male patients older than 50 years.25 They excluded those considered unfit for potential AAA repair. The remaining patients were invited for ultrasound screening and had an attendance rate of 74%. A significant (49%) reduction in future ruptured AAAs was noted in patients who were invited. Although only 74% to 84% of patients were screened in these two studies, detection of small AAAs and subsequent management reduced rupture risk by half in the entire group of patients invited for AAA screening.
Randomized Trials
The first randomized trial of AAA screening enrolled 6058 unselected 65- to 80-year-old men in Chichester, United Kingdom, 74% of whom attended screening.77 AAAs (≥3 cm) were detected in 7.6%. AAA-related death was subsequently reduced by 41% at 5 years and 21% at 10 years.78 The investigators also enrolled 9342 unselected 65- to 80-year-old women, 65% of whom attended screening.79 The prevalence of AAA in women was just 1.3%. Women did not seem to benefit from screening because there was no difference in elective AAA repair, ruptured AAA, or AAA-related mortality at 5 or 10 years in women offered screening. The average age of women with a ruptured AAA was 6 years older than men. For both men and women, it is important to note that the comparison was between those offered screening and those who were not offered screening. The Chichester group noted that a substantial number of ruptured AAAs occurred in patients invited for screening who refused screening, did not adhere to follow-up, refused surgery, or were considered unfit for surgery after AAA was diagnosed.
In the second randomized trial of AAA screening, all 12,658 65- to 73-year-old men in Viborg County, Denmark, were randomized.80 The attendance rate was 76%. AAA (≥3 cm) was diagnosed in 4%. In-hospital AAA-related mortality was reduced by 68% in men invited for screening (P < .01). Outpatient deaths were not recorded, however. Additionally, studies reporting simply a reduction in AAA-related deaths fail to account for the timing of death. Patients who die as a result of elective surgery for screening-detected AAAs tend to die sooner than patients who die as a result of rupture of an undiagnosed AAA, thus resulting in a greater loss of life-years per death in the screened group.
The Multicentre Aneurysm Screening Study (MASS) was a well-designed, carefully conducted, and thoughtfully analyzed randomized trial that involved 70,495 men, ages 65 to 74 years, and excluded 4% who were considered unfit for possible surgical repair, finally randomizing 67,770 men.81 Follow-up was to 13 years postrandomization. Of the 33,839 patients randomized to ultrasound screening (versus no screening), 80% accepted and were scanned. The aorta was not visualized in 1.2%, and AAAs larger than 3 cm were detected in 5%. The 33,961 control patients were not contacted. At the close of the trial, after 13 years follow-up, there were 381 AAA-related deaths in controls and 224 in the invited group, a relative risk reduction of 42% (hazard ratio [HR], 0.58; 95% CI, 0.49-0.69) (Fig. 130-7). The incidence of nonfatal ruptured AAAs was similarly reduced in the invited group (HR, 0.57; 95% CI, 0.49-0.66). Although there were more deaths as a result of elective AAA repair in the screened group (24 versus 14), this was more than offset by the reduction in deaths from ruptured AAAs (327 versus 170). As well as these reductions in aneurysm-related mortality and nonfatal aneurysm rupture, there was a 3% reduction in all-cause mortality ( HR 0.97; 95% CI, 0.95-0.99).82
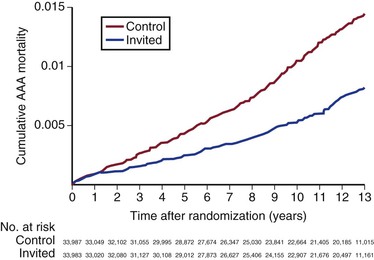
Figure 130-7 Final results of the Multicentre Aneurysm Screening Study (MASS) trial demonstrating a highly significant reduction in aneurysm-related mortality in the group invited for abdominal aortic aneurysm (AAA) screening (hazard ratio 0.58; 95% confidence interval, 0.49-0.69). (From Thompson SG, et al: On behalf of the Multicentre Aneurysm Screening Study (MASS) Group: Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg 99:1649-1656, 2012.)
A meta-analysis in which the long-term results from all the major screening studies were analyzed was large enough (with more than 125,000 patients) to demonstrate not only a 44% reduction in the AAA-related mortality rate but also a small but statistically significant reduction in overall mortality in the long-term (7- to 15-year) results.83
Factors Affecting Clinical Decision Making
The choice between observation and prophylactic surgical repair of an AAA for an individual patient at any given time should take into account (1) the risk for AAA rupture under observation; (2) the operative risk associated with repair, and by inference, the modality and location of repair; (3) the patient’s life expectancy; and (4) the personal preferences of the patient.84,85
Aneurysm Rupture Risk
Once an AAA has been diagnosed, either by chance or through a screening program, the first key question is to define an estimate of aortic rupture risk for that individual patient. Unfortunately, estimates of the rupture risk remain imprecise because, fortunately, large numbers of patients with AAAs have not been observed to the point of rupture without intervention. Even in recent randomized trials of surveillance versus early surgical repair, the majority of patients in the surveillance arms eventually underwent repair, for a number of reasons, thus underestimating rupture risk.
Studies conducted before the widespread application of surgical repair documented the likelihood of large AAAs to rupture, although many of these AAAs were both large and symptomatic, thus overestimating rupture risk.86,87 Even autopsy studies46,88 may underestimate the diameter at rupture because the aneurysm is no longer pressurized at autopsy, and rupture risk may be overestimated because there is no way of knowing how many patients with asymptomatic aneurysms did not undergo autopsy. Varying populations, referral patterns, surgical intervention rates, imaging methods, and definitions of how to measure diameter lend further variability to the data, as evidenced by the wide range of potential rupture risk in the literature for a given aneurysm size (Table 130-4). To date, the data have been insufficient to develop an accurate prediction rule for AAA rupture in individual patients, although there is ongoing work to provide exactly this. If successful, when combined with a surgical risk algorithm, the decision to operate might become considerably easier as both individualized rupture risks and operative risks will be available to the surgeon and the patient. With the current state of knowledge, however, surgeons rely on the output of large-scale randomized trials into the management of small aneurysms and extrapolations of these and other studies to estimate the natural history of any given aneurysm.
Table 130-4
Range of Potential Rupture Rates for a Given Size of Abdominal Aortic Aneurysm
| AAA Diameter (cm) | 12-Month Rupture Risk (%) |
| 3.0-3.9 | 0.3 |
| 4.0-4.9 | 0.5-1.5 |
| 5.0-5.9 | 1-11 |
| 6.0-6.9 | 11-22 |
| >7 | >30 |
AAA, Abdominal aortic aneurysm.
Therefore, the most widespread estimation of rupture risk remains aneurysm diameter, which is taken in consideration with aneurysm morphology and aneurysm growth rate. Each of these is discussed along with the potential utility of wall stress analysis, a technique that is currently limited to a research tool, but shows promise.
Effect of Aneurysm Diameter
The primary determinant of rupture risk is the maximum aneurysm diameter, based on a pivotal study reported by Szilagyi et al in 1966.89 These authors compared the outcomes of small- and large-diameter aneurysms and found that patients with larger aneurysms (>6 cm) were much more likely to die of a ruptured aneurysm. The results were supported in 1969 by a study reporting rupture in 16% of AAAs less than 6 cm in diameter versus rupture in 51% of AAAs greater than 6 cm in patients managed nonoperatively.90 In these series, AAA diameter was determined by physical examination and abdominal radiography, both of which are now known to overestimate actual diameter, such that the actual size for their definition of a 6-cm AAA was closer to 5 cm. Early autopsy studies also demonstrated that larger AAAs are more prone to rupture.46 One series found that rupture had occurred in 5% of AAAs 5 cm or less in diameter, in 39% of AAAs 5 to 7 cm in diameter, and in 65% of AAAs 7 cm or greater in diameter.88 These studies firmly established the effect of size on AAA rupture and provided a sound basis for recommending elective repair for large AAAs because even these early studies showed a marked improvement in survival after operative repair.89,90
In a Minnesota population-based study of 176 patients with AAAs selected for nonoperative management, the estimated annual rupture risk was zero for AAAs less than 4 cm, 1% per year for AAAs 4.0 to 4.9 cm, but 11% per year for AAAs 5.0 to 5.9 cm.91 These rates also probably underestimate rupture risk, however, because 45% of AAAs underwent elective repair during follow-up, presumably those at greatest risk for rupture within any size category.91 In another study of 114 patients with small AAAs initially selected for nonoperative management, Limet et al92 observed rupture in 12% during a 2-year follow-up despite elective repair in 38% because of rapid expansion. This yielded an annual rupture rate of zero for AAAs less than 4 cm in diameter, 5.4% per year for AAAs 4 to 5 cm in diameter, and 16% per year for AAAs more than 5 cm in diameter. Because this was a referral-based study, it probably overestimated rupture risk for the entire population, but may accurately portray the group of patients referred for surgical consultation. However, in another referral-based study of 300 patients with AAAs initially managed nonoperatively, the observed annual rupture risk during a 4-year follow-up was only 0.25% per year for AAAs less than 4 cm, 0.5% per year for AAAs 4 to 4.9 cm, and 4.3% per year for AAAs more than 5 cm in diameter, although only 8% of the patients underwent elective repair.93
Estimates from Recent Trials
United Kingdom Small Aneurysm Trial.
Data from the United Kingdom Small Aneurysm Trial (UKSAT) provided the most comprehensive estimates of AAA rupture risk in patients randomized to nonoperative management. The UKSAT was the first randomized trial to compare early surgery with surveillance for AAAs 4 to 5.5 cm, and it enrolled 1090 patients ages 60 to 76 years.94 Patients who underwent surveillance were monitored by repeat ultrasound every 6 months for AAAs 4 to 4.9 cm and every 3 months for AAAs 5 to 5.5 cm. If AAA diameter exceeded 5.5 cm, the expansion rate was more than 1 cm per year, the AAA became tender, or repair of an iliac or thoracic aneurysm was needed, elective surgical repair was recommended. At the initial report in 1998, after a mean 4.6 years of follow-up, there was no difference in survival between the two groups. Survival was initially worse in the early surgery group because of perioperative mortality, which was unexpectedly high at 5.8%. After 3 years, patients who had undergone early surgery had better late survival, but the difference was not significant. Operative mortality was 5.8% in the early surgery group and 7.2% in the surveillance group, which included more emergency and urgent repairs than in the early surgery group.
The annual rupture risk was 0.3% for AAAs ≤3.9 cm, 1.5% for AAAs 4.0 to 4.9 cm, and 6.5% for AAAs 5.0 to 5.9 cm.95 These numbers do not accurately represent the rupture risk for women, who made up only 17% of the trial and had a 4.5-fold higher risk for rupture than men did. It is also likely that these numbers underestimate the actual annual rupture risk for small AAAs because a number underwent repair for rapid expansion or the development of symptoms. There is some evidence that in patients who will live for a long period, particularly young patients (60-64 years; HR, 0.73: 95% CI, 0.55-0.99), or in patients with larger AAAs (4.9-5.5 cm; HR, 0.79; 95% CI, 0.60-1.04), there is a trend toward a benefit of early surgery, but overall no difference was observed (Fig. 130-8). At the close of the trial follow-up, more than 75% of the surveillance group had undergone aneurysm repair (Fig. 130-9).
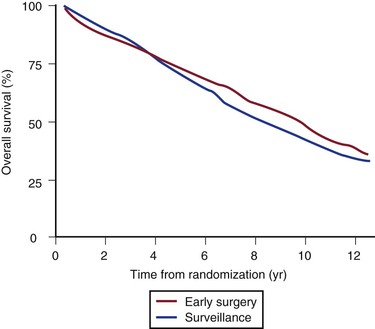
Figure 130-8 Final results from the UK Small Aneurysm Trial showing no difference survival in the early surgery versus surveillance group. At 8 years, there was a small survival advantage in the early surgery group (P = .02), but at the completion of the trial there was again no difference due to deaths from comorbidity. (From Powell JT, et al: Final 12-year follow-up of surgery versus surveillance in the UK Small Aneurysm Trial. Br J Surg 94:702-708, 2007.)
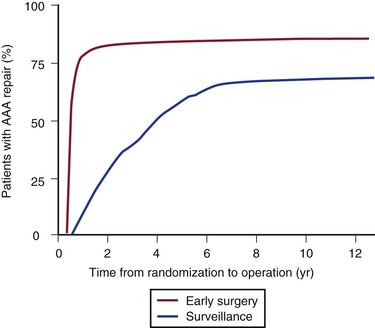
Figure 130-9 Final results from the UK Small Aneurysm Trial showing the proportion of patients in both the early surgery group and the surveillance group eventually undergoing aneurysm repair. Three quarters of the surveillance group eventually had aneurysm repair, with a 30-day surgical mortality of 7.2% versus 5.5% in the early surgery group (P = .283). AAA, Abdominal aortic aneurysm. (From Powell JT, et al: Final 12-year follow-up of surgery versus surveillance in the UK Small Aneurysm Trial. Br J Surg 94:702-708, 2007.)
In long-term follow-up,96 there was a survival advantage in the early surgery group at 8 years after randomization (7.2% improved survival; P = .020), but a high all-cause mortality late in the trial attenuated this advantage to become nonsignificant again by 12 years. Fatal rupture occurred in 5% of men but in 14% of women, such that risk of rupture was approximately three times higher for women. This finding prompted a recommendation of a lower diameter threshold for elective AAA repair in women (5 cm). A separate analysis of the UKSAT showed that a strategy of early surgery for small AAAs was more costly but associated with small gains in health-related quality of life at the latter analysis.97
Aneurysm Detection and Management Study.
The Aneurysm Detection and Management (ADAM) study, conducted at U.S. Veterans Affairs (VA) hospitals, was published in 2002.98 In this trial, 1163 veterans (99% male) ages 50 to 79 years with AAAs 4 to 5.4 cm were randomized to early surgery versus surveillance. Surveillance entailed ultrasound or CT every 6 months with elective surgery for AAA expansion to 5.5 cm, expansion of 0.7 cm in ≥6 months, expansion of more than 1 cm in 1 year, or development of symptoms attributable to the AAA. Patients with severe heart or lung disease were excluded, as were patients who were not thought to be likely to comply with surveillance. As in UKSAT, there was no survival difference after a mean follow-up of 4.9 years. Similarly, more than 60% of patients in the surveillance arm underwent repair. Initial AAA diameter predicted subsequent surgical repair in the surveillance group: 27% of patients with AAAs initially 4 to 4.4 cm underwent repair during follow-up compared with 53% of patients with AAAs 4.5 to 4.9 cm and 81% of patients with AAAs 5 to 5.4 cm. Operative mortality was 2.7% in the early surgery group and 2.1% in the surveillance group. The ADAM study confirmed the results of the UKSAT by showing a lack of benefit of early surgery for AAAs 4 to 5.5 cm, even if operative mortality was low and compliance with surveillance was high, because most patients ultimately require surgery as their AAA expands.
Meta-Analysis of Trials.
Individual patient data meta-analysis from 18 studies (15,475 participants) investigating the surveillance of small aneurysms or aneurysm screening was undertaken in the RESCAN study to determine factors influencing the growth and rupture rate of small aneurysms.99 This study, which included individual patient data from UKSAT, ADAM, and the American Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) trial, confirmed that there were a number of independent factors that affected aneurysm growth rate, most notably current smoking, which increased the growth rate by a mean ± SEM 0.354 ± 0.065 mm/year, and diabetes, which reduced the growth rate by −0.505 ± 0.097 mm/year. In terms of small aneurysm rupture, there was a strong association between current smoking and rupture (pooled HR, 2.02, 95% CI, 1.33-3.06; P = .001). Women also had a much higher risk of rupture than men (pooled HR, 3.76, 95% CI, 2.58-5.47; P < .001). Smaller effects were seen for older individuals and patients with hypertension, patients with a lower body mass index, and patients with a wider pulse pressure.
Small Abdominal Aortic Aneurysms: Impact of Endovascular Aneurysm Repair
It is plausible that, with the routine availability of EVAR, much lower operative mortality rates may have altered the risk-benefit balance of operating on small aneurysms compared with open repair. To address this, two randomized trials were conducted: Comparison of Surveillance Versus Aortic Endografting for Small Aneurysm Repair (CAESAR) and PIVOTAL. Each of these trials randomized patients to either surveillance of small AAAs or early EVAR. If these trials showed different results compared with UKSAT or ADAM, then considerable change would occur in treatment algorithms for AAA and huge increases in the operative workload of vascular units.
CAESAR Trial.
The CAESAR trial100 randomized 360 patients with aneurysms of 4.1 to 5.4 cm to either immediate EVAR or surveillance and repair at defined threshold events (diameter growth to >5.5 cm, aneurysm enlargement >1 cm/year, development of symptoms) between 2004 and 2008. The perioperative mortality was 0.55%, far lower than in the intervention arm of UKSAT or ADAM.95,98 After 54 months median follow-up, there was no difference in all-cause mortality or aneurysm-related mortality between groups (14.5% vs 10.1%, EVAR vs surveillance).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree