Chapter 133
Abdominal Aortic Aneurysms
Ruptured
Manish Mehta, John Byrne
Based on a chapter in the seventh edition by Thomas F. Lindsay
Most patients with a ruptured abdominal aortic aneurysm (RAAA) die before they can be operated upon.1,2 Furthermore, surgery carries a high mortality3 and consumes an inordinate amount of health care resources.4 However, treatment is changing. After decades of marginal improvements in open surgical survival rates,5–7 there has been a paradigm shift. Endovascular techniques, advances in perioperative management, and centralization of services have improved outcomes, with substantial reductions in mortality in some regions.8,9 Ironically, this has coincided with a declining incidence of RAAA in many developed countries.10–13
Terminology and History
Definitions
RAAA is an abdominal aortic aneurysm (AAA) with extraluminal blood on computed tomography (CT) or noted clinically at the time of surgery. A contained rupture refers to blood outside the aneurysm sac that is confined to the retroperitoneal space, tamponaded by the surrounding tissues. A free rupture refers to bleeding into the peritoneal cavity, without tamponade. A symptomatic, nonruptured AAA is one with back pain or tenderness over the aorta on deep palpation but with an intact aneurysm on CT. The pain is thought to be related to acute expansion of the wall, intramural hemorrhage, wall degeneration, or bleeding into the thrombus and is therefore considered a prelude to actual rupture, which requires urgent repair. Symptomatic aneurysms are not associated with hypotension; however, the prognosis is much better than that of RAAAs (but worse than after elective repair).14 The inclusion of symptomatic aneurysms in data on RAAAs will artificially improve the data. Those with symptoms and an AAA require rapid diagnosis and management to prevent rupture and its associated adverse outcomes.15
History
The first successful reconstruction for RAAA was by Henry Bahnson with a homograft in 1953.16 Before that, the only treatment was aortic ligation. The first reported operation was in 1817 by Astley Cooper, who ligated the aortic bifurcation in a 38-year-old man for a ruptured left external iliac artery aneurysm (29 years before general anesthesia).17 The patient died on the second postoperative day. Another 11 aortic ligations were attempted before the end of the 19th century; none was successful. Surgeons in the early part of the 20th century fared little better despite using a variety of different materials for ligation (soft rubber catheters, aluminum bands, catgut). Eventually, in 1923, Rudolph Matas successfully ligated the aorta of a 28-year old “field laborer” with a ruptured syphilitic aortic aneurysm.18 She survived 28 months, succumbing to tuberculosis. Despite this, ligation was not widely embraced, and definitive surgical reconstruction was established after 1953. By 1954, Cooley and DeBakey had treated six patients with a 50% survival rate.19 Javid and colleagues from Chicago reported another four cases the following year, also with a 50% survival rate, and the Mayo Clinic reported its first two cases in 1956.20 Just as elective open aneurysm reconstruction was rapidly applied to ruptures, so too was endovascular aneurysm repair (EVAR). Following the first description of EVAR in 1991, Hopkinson performed the first EVAR for rupture in Nottingham, England, in 1994.21 This, too, has been widely adopted.
Epidemiology
Declining Incidence
Reported incidences of RAAA have fluctuated during the last 6 decades. In the 1950s and 1960s, diagnosis was made on clinical grounds (or at autopsy), without CT or other abdominal imaging, which resulted in a low reported incidence. Contemporary data are more reliable. From 1999 to 2009, the Centers for Disease Control and Prevention reported 49,207 deaths from RAAA in the United States,22 with most deaths in the 75- to 84-year age group. This excluded those undergoing successful surgery. Until recently, the incidence of RAAA was thought to be increasing annually. In 2006, a population-based report from Sweden showed that the incidence of RAAA almost doubled between 1971 and 2004.23 In the United States, between 1951 and 1980, the incidence of AAA rose by sevenfold.24 More specifically, the incidence of RAAA in men increased threefold despite more elective repairs from 1971 to 2004. However, in 2011, a decline in the incidence of ruptured (and intact) AAA began to be reported in most Western populations (Fig. 133-1). In Australian men, the number of deaths from RAAAs fell by 38% between 1999 and 200625; in New Zealand, the decline during a similar period was 53%.26 In 2012, a review of population-based mortality and hospital admissions in England and Wales from 1979 to 2009 showed a steady increase in deaths from RAAAs, especially in men, until 1996.10 Then, deaths began to rapidly decline. By 2009, the mortality rate was lower than in 1979. In the United States, this phenomenon has also been observed. In 2012, a retrospective study of 338,278 Medicare beneficiaries from 1995 to 2008 reported that AAA-related deaths halved during the study period, with the greatest decline seen in patients older than 80 years.11
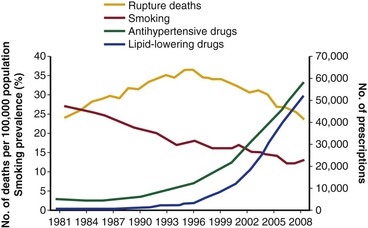
Figure 133-1 Changes in deaths from abdominal aortic aneurysm rupture, prevalence of smoking among the total population older than 65 years, and prescription of blood pressure– and lipid-lowering drugs in England and Wales, 1981-2009. (From Anjum A, et al: Explaining the decrease in mortality from abdominal aortic aneurysm rupture. Br J Surg 99:637-645, 2012.)
The reason for the declining incidence of RAAA is not clear. It cannot be explained by improved surgical outcomes for RAAA in large centers of excellence. This is because most deaths from RAAA occur before a patient reaches the hospital, so that overall mortality rates from RAAAs remain between 74% and 90%.1,2,27–29
Population screening has been shown to reduce AAA-related deaths,30,31 but the decline in mortality due to RAAA began before population screening became widespread. The availability of EVAR may have led to more elective AAA repairs in older patients and those who would previously have been considered unfit for open surgery, which could have reduced the at-risk population for RAAA.32 However, the biggest reduction in absolute numbers of ruptured aneurysms has come from a decline in the prevalence of intact AAA. In 2011, Svensjö et al33 reported that the prevalence of screen-detected AAAs in the Swedish screening program was “lower than expected” at 1.7%. The U.K. screening program detection rate is now also only 1.7%, much less than the 4% detection rate expected when the program was initiated.34 In the county of Gloucester in England, where population screening has been taking place since 1990, the prevalence has dropped from 4.8% to 1.1% in 65-year-old men.35 This has been attributed to decreased levels of smoking (see Chapter 27).
Mortality of Ruptured Abdominal Aortic Aneurysm
In 1988, an 8-year survey in the southeast of England reported a community mortality rate of 89% for RAAAs.27 Two thirds of patients failed to reach the hospital alive. Another 1988 study from the southwest of England reported a remarkably similar 86% community mortality rate.28 In 2006, a population-based Swedish study23 with a high autopsy rate reported that the early mortality rate (death in the community or during hospitalization) was 74% and that, as recently as 2004, only 48% of patients underwent repair.
Data from the Danish national registry from 1994 to 2008 show the overall mortality risk from RAAA to be 76%. Again, less than half underwent surgery.36 Postoperative mortality decreased from 51% to 42%. This was attributed to centralization of services and better prehospital care. In 2013, the Swedish vascular database (Swedvasc) also reported an improvement in outcomes for patients with RAAAs.37 Data of 4972 patients with ruptured aneurysms from 1994 to 2010 were reviewed and showed a decline in mortality from 38% to 28%, which was credited to the introduction of EVAR. The most ambitious review of population data was by Mani et al38 using the Vascunet database. In 2011, they collated outcomes for RAAA repair in 7040 patients from national and regional registries in nine countries (Australia, Denmark, Finland, Hungary, Italy, Norway, Sweden, Switzerland, and the United Kingdom). The overall mortality rate was 31.6%, but it declined by 4.2% between 2005 and 2009. This was again attributed to more liberal use of EVAR.
Mortality of Ruptured Abdominal Aortic Aneurysm Repair
A meta-analysis of the first 50 years of RAAA publications noted that the mortality rate for ruptured repair has fallen only 3.5% per decade since the initial successful repairs were reported.7 A meta-analysis of publications between 1991 and 2006 suggested no significant overall change in mortality with open surgical repair (OSR) during this period.5 This conclusion is supported by data from U.S. Veterans Affairs hospitals and the U.S. National Hospital Discharge Survey database (for the years 1979 to 1997), which have not demonstrated a decline in RAAA mortality.7 Between 1994 and 2003, mortality after OSR of RAAA was unchanged on the basis of U.S. Medicare data.39
The high postoperative mortality rates after aortic rupture are secondary to a high incidence of myocardial infarction, renal failure, and multiple organ failure (MOF). Aortic rupture and OSR result in a combination of ischemia-reperfusion injuries, hemorrhagic shock, and lower torso ischemia. The synergistic effect of the total-body ischemia caused by hemorrhagic shock and the lower torso ischemia that occurs during repair, followed by reperfusion secondary to resuscitation and aortic unclamping, has been proposed to explain the high incidence of MOF and mortality after repair. This hypothesis is supported by data from both animal experimentation and human clinical studies.40,41 The application of endovascular techniques to repair of RAAAs (EVAR) reduces the operative and ischemic insult in two ways. First, it avoids a laparotomy, thus reducing the potential for iatrogenic arterial and venous operative injuries and their associated blood loss. Second, it minimizes the duration and severity of the lower torso ischemic insult. Recent data from large administrative databases in the United States have demonstrated increased application of EVAR to RAAA.39,42–44 Analysis of mortality rates of EVAR cases between 2000 and 2003 noted a significant reduction in the mortality of RAAA patients treated by EVAR (31.85 vs. 50.8%; P < .001).45
Pathophysiology
Aneurysm rupture represents failure of the aortic wall to bear the load or tension to which it is exposed. When the force of the blood pressure exceeds the ability of the aortic wall to bear that load, the aneurysm wall ruptures and blood extravasates. In vitro testing has demonstrated that the failure strength of human aneurysmal aortic wall is 65 N/cm2 versus 121 N/cm2 for nonaneurysmal human aortic wall. This indicates that the aneurysmal aortic wall is a weak structure.46,47 Laplace’s law (tension is proportional to radius times pressure) relates wall tension to pressure, thus emphasizing the importance of size. Although this method may be satisfactory for symmetrical structures, it does not take into account the complex geometry of individual AAAs.
Abdominal Aortic Aneurysm Shape and Finite Element Analysis
The engineering technique of finite element analysis has been applied to AAAs to estimate regional variations in peak wall stress throughout the entire aneurysm wall. With use of CT scan data, a mesh is applied to the aortic wall, and finite element analysis is used to calculate peak wall stress for many small segments of the aortic wall to provide a visual representation of peak wall stress (Fig. 133-2). Early three-dimensional representations of peak wall stress noted that the posterior wall of the aorta had higher values corresponding to the site where most AAAs were noted in clinical observations and autopsy studies to have ruptured.48,49
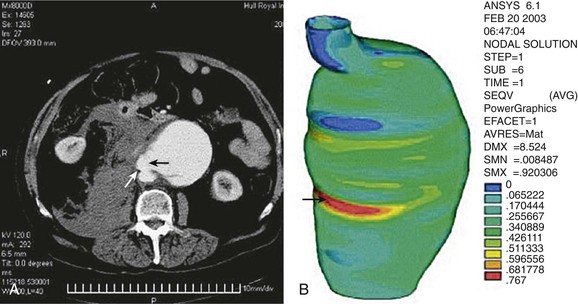
Figure 133-2 Point of rupture in CT (A) correlated with area of high stress (B) in finite element analysis. Site of rupture and area of high stress are indicated by arrows. (From Venkatasubramaniam AK, et al: A comparative study of aortic wall stress using finite element analysis for ruptured and non-ruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 28:168-176, 2004.)
Initial studies assumed that AAAs had uniform wall thickness and material isotropy (uniform strength in all orientations), but several studies50–52 show this is not the case. There are localized “hot spots” of matrix metalloproteinase (MMP) activity that could cause focal weakening of the aneurysm and rupture at relatively low levels of intraluminal pressure. Newer models include growth and remodeling theory that allows evolving wall thickness, stiffness, regional heterogeneity, and fluid-structure interactions within the aneurysm sac to be analyzed.53,54 As a result, we may not be far off from the day when individual risk of rupture can be accurately estimated from conventional CT scans.
Role of Aortic Thrombus
In 1965, Martin55 surmised that as an aneurysm develops, it becomes lined with thrombus, which weakens it by interfering with nutrition of that segment of the wall. Contemporary studies confirm this, showing that intraluminal thrombus is not protective and weakens the aneurysm wall.56 In 2000, using a pressure transducer during open aneurysm repair, Schurink et al57 confirmed that intraluminal thrombus does not reduce pressure near the aneurysm wall. Vorp et al58 also confirmed localized hypoxia in regions of thicker thrombus that might lead to localized wall neovascularization, inflammation, and regional wall thinning. Hypoxia also affects the function of vascular smooth muscle cells, causing them to secrete more collagenase than in normoxic conditions, with less elastin and collagen production.
Aneurysm thrombus is also not inert. MMPs are a family of 28 endopeptidases that comprise collagenases, gelatinases, and stromelysins. They degrade extracellular tissues, cleave cell surface receptors, and are involved in tissue remodeling. They are inhibited by four endogenous tissue proteins called tissue inhibitors of metalloproteinases. Intraluminal thrombus contains high levels of MMP-2 and MMP-9, trapped neutrophils rich in MMP-9, and neutrophil gelatinase-associated lipocalin, which neutralizes the defensive actions of tissue inhibitors of metalloproteinases. An autopsy study of 78 patients who died of infrarenal RAAAs showed that 80% of ruptures occurred at the site of mural thrombus.59 This was supported by CT studies of patients admitted with rupture, which showed that most ruptures occurred through the thrombus or at its edge. Others argue that cracks or fissures in the intraluminal thrombus due to proteolytic enzymes on the luminal surface of the clot are what really cause rupture.60 However, consensus seems to be building. A Dutch report61 in 2013, while accepting a role for fissure formation, confirmed that intraluminal thrombus thickness was associated with vascular smooth muscle cell apoptosis (or cell death), elastin degradation, and high levels of MMP-2 and correlated with aneurysm rupture. (Details of aneurysm wall biology are discussed in Chapter 9).
Clinical Features
Signs and Symptoms
The classic presentation of RAAA is a male patient older than 60 years complaining of acute-onset abdominal and back pain. Back pain is caused by rupture of the aneurysm into the retroperitoneal space and is severe and unremitting. Patients may describe pain radiating to the testes, inguinal canal, or rectum. Some patients may describe hip pain due to psoas irritation. On examination, the patient is usually pale, diaphoretic, and hypotensive with a tender, expansile abdominal mass. Rarely, there may be flank ecchymosis due to tracking of retroperitoneal blood. There may be documentation of a prior diagnosis of AAA.
Differential Diagnosis
In patients older than 50 years with hypotension or syncope (or both), consideration of RAAA is critical because it has the worst prognosis of conditions included in the differential diagnosis. A rapid, accurate history and examination are critical. The differential diagnosis may include renal colic, diverticulitis, pancreatitis, gastrointestinal hemorrhage, inferior myocardial infarction, and perforated ulcer. Although the signs and symptoms of RAAA can be clear-cut, it may be difficult to recognize in the early stages. Many patients will initially have relatively normal blood pressure on arrival in the emergency department, after fluid resuscitation en route. Some may give a history of pain extending during the course of 3 or 4 days as the AAA has bled, sealed, and then bled again. In the affected age group, chronic back problems are common, so the physician may be tempted to ascribe symptoms to an exacerbation of a long-standing problem. The other misdiagnosis is to confuse the presentation with ureteral colic. Symptoms may be incorrectly attributed to acute diverticulitis (especially if leukocytosis is present) or a perforated duodenal ulcer. Rare presentations include rupture into an adjacent structure, such as an acute gastrointestinal bleed due to a primary aortoduodenal fistula or an aortocaval fistula that presents as an expansile abdominal mass, loud machinery-like bruit, or thrill with new-onset acute congestive heart failure from a high-output fistula.
Studies have examined the clinical findings in patients in whom RAAA was subsequently diagnosed.62 In only 23% was a definitive and immediate diagnosis of RAAA made by the first physician who examined the patient.63 The rate of incorrect diagnosis ranges from 16% to 60%.64 The most common misdiagnoses were renal colic, perforated viscus, diverticulitis, gastrointestinal hemorrhage, and ischemic bowel. The initial manifestation of renal colic occurs infrequently in patients older than 50 years. The classic triad of pain, hypotension, and pulsatile mass was present in only 9% of the misdiagnosed group compared with 34% of the correctly diagnosed group. The presence of a pulsatile mass was identified in 72% of those correctly diagnosed but in only 26% of the misdiagnosed patients. Thus, the lack of a pulsatile mass frequently confuses the diagnosis. Furthermore, the use of ultrasound in older studies was reported as being consistent with rupture in only 51% of cases.64
Diagnostic Evaluation
Plain Radiographs
Plain radiography is now less frequently performed than ultrasound or CT in patients with abdominal pain. However, if the aortic wall is sufficiently calcified, an aneurysm not otherwise suspected can be identified on radiographs. A retrospective review of plain films of patients with RAAAs noted evidence of the diagnosis on 90% of the films.65 Enlargement of a calcified aortic wall beyond normal limits was seen in 65%, and loss of a psoas shadow from retroperitoneal hemorrhage was identified in 75%.
Ultrasound
The introduction of bedside ultrasound in the emergency department and training of emergency physicians in its application for trauma have led to its use for imaging of the abdominal aorta in cases of suspected rupture. FAST (focused assessment with sonography in trauma) ultrasound protocols can rapidly identify fluid collections and direct further trauma care.66 It has been used in the emergency department to rapidly evaluate aortic diameter in those with a suspected RAAA.67 Ultrasound examination of the abdominal aorta can accurately identify an AAA and frequently takes less than 5 minutes.68 When ultrasound is used in patients with back pain, abdominal pain, or other suggestion of AAA, it can identify aortic aneurysms with high sensitivity, specificity, and positive and negative predictive values.69 To date, no large prospective studies have been conducted; however, it appears that with appropriate training of emergency physicians, the use of ultrasound to quickly assess patients for the presence of AAA is probably beneficial because it can rapidly direct further consultation, investigation, and therapy. Although ultrasound can rapidly identify an AAA, it is not accurate for exclusion of rupture.68,69
Computed Tomography
Accuracy.
The most accurate method for diagnosis of RAAA is CT, which also provides important information about associated abnormalities and conditions. CT scanning in patients thought to have RAAA or other causes of abdominal pain is frequently ordered by the emergency physician when the diagnosis is unclear. A non–contrast-enhanced CT scan can identify retroperitoneal hemorrhage associated with RAAA and can also provide important anatomic information (Fig. 133-3). In addition, alternative abdominal disease may be discovered should no AAA be identified. CT scanning with contrast enhancement is required for ideal planning of RAAA repair with commercially available stent-graft devices for the same reasons that it is required for elective repair. The volume of contrast material required can be reduced with proper timing, which can lessen its renal impact, especially if EVAR is performed with additional volumes of contrast agent. Renal dysfunction may be induced by the combination of high doses of contrast material and renal hypoperfusion.
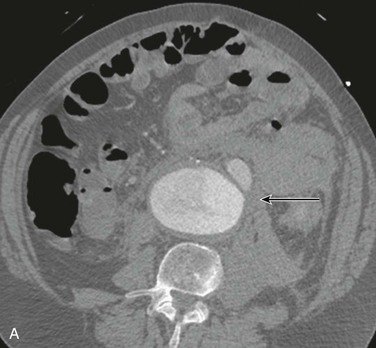
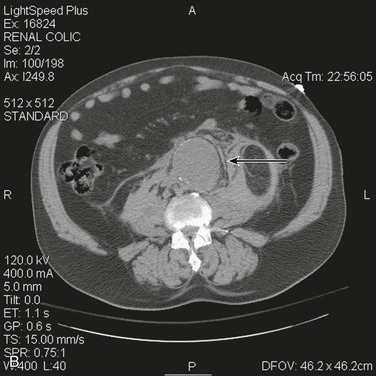
Figure 133-3 A, Frank rupture of an infrarenal abdominal aortic aneurysm with contained extravasation (arrow). B, CT scan of a ruptured abdominal aortic aneurysm. Note the pattern of stranding of blood into the tissues (arrows). No contrast material was used, and the scan was performed for the diagnosis of renal colic.
Evidence of retroperitoneal blood, used as the “gold standard” to diagnose RAAA on CT, was found to be 77% sensitive and 100% specific in a retrospective series.70 Its positive predictive value was 100% and its negative predictive value was 89%. The low rate of false-negative studies (no rupture on CT but RAAA found at surgery) is comforting. Interestingly, the level of agreement of the diagnosis of RAAA based on CT scanning was only moderate in five observers, whereas agreement on suitability for EVAR was only fair in vascular surgeons and radiologists experienced in EVAR from the Amsterdam Acute Aneurysm study.71 Intraobserver agreement on rupture and suitability for EVAR was higher than interobserver agreement. This disconcerting finding remains to be confirmed by others.
Timing.
Prospective and retrospective studies have examined the delay between the initial manifestation of RAAA and the time to death to determine whether sufficient time exists to perform a CT scan and to evaluate patients’ suitability for EVAR. Patients who were not treated surgically for RAAA because of comorbidity or advanced age had a median time from onset of symptoms to death of just more than 16 hours.72 Only 13% died within 2 hours of hospital admission, and the median time between admission and death was 11 hours. This suggested that sufficient time exists for most patients to undergo CT scanning and evaluation for EVAR. A prospective analysis from regional U.K. centers identified that 33% of RAAA patients were transferred from other hospitals. The median time from arrival to surgery for operative cases was 159 minutes, and mortality was not affected by the length of delay or CT scanning. Thus, for the majority of patients who are stable on arrival at a center where definitive care will be carried out, a prompt CT scan does not appear to adversely affect mortality.73
Although studies show that on average there is sufficient time between arrival at the emergency department and death from RAAA for performance of CT, it is difficult to predict continued hemodynamic stability of individual patients during a CT scan. Thus, CT scans should be performed with a minimum of delay. The patient should be monitored and accompanied at all times because deterioration can occur rapidly. Both the U.K. RAAA randomized trial and the Amsterdam Acute Aneurysm study identified patients too unstable for CT scanning who required immediate transfer to the operating room.74,75 In the U.K. RAAA randomized trial, the time to arrival at the operating room was 25 minutes shorter in the EVAR group versus OSR despite the requirement for a contrast-enhanced CT scan. This suggests that the existence of a protocol for management of RAAA may significantly shorten the time to definitive therapy despite the requirement for the scan.76,77
Initial Management Strategies
Patient Triage and Transfer to Appropriate Institutions
RAAA is a vascular surgical emergency, and when the diagnosis is being considered, immediate vascular surgery consultation is critical. Hospitals that lack vascular surgery expertise and cannot offer definitive treatments should consider establishing networks with larger institutions that have vascular surgery services and can offer repair by endovascular or open surgical means, as needed. The impact of transferring RAAA patients to regional centers where repair can be carried out by experienced vascular teams has been studied. Rural hospitals without vascular surgery expertise tend to face a higher burden of RAAA patients, and national trends suggest that they are more likely to transfer patients to regional centers.78 Although patient transfer might increase the time of arrival from the initial hospital to the operating room, most studies suggest that high-volume regional centers significantly lower the mortality for RAAA patients.79 Furthermore, as U.S. nationwide trends suggest increased use of EVAR for RAAA, it is likely that regionalization of patient care will have a significant impact on management of RAAA patients.80
Permissive Hypotension
Preoperative resuscitation of RAAA patients with hypotension must be judicious.81–84 Large volumes of intravenous fluids and a rise in blood pressure can lead to further hemorrhage by overcoming the tamponade that stabilized the initial rupture. It also causes hemodilution, coagulopathy, hypothermia, and acidosis, and further deterioration.85 Similar to transfusion protocols that restrict aggressive fluid resuscitation in hypotensive trauma patients with hemorrhagic shock, permissive hypotension for RAAA patients is recommended. Fluid resuscitation is minimized to maintain consciousness, to prevent ST depression, and usually to maintain a systolic pressures of 70 to 80 mm Hg.86 Crawford initially suggested minimal fluid resuscitation to maintain systolic blood pressure at 50 to 70 mm Hg in patients with RAAA.82 Although, all contemporary algorithms for management of RAAA patients include permissive hypotensive resuscitation,87 a balance must be sought between organ ischemia from inadequate resuscitation and the risk of rebleeding from overaggressive resuscitation. Aggressive volume resuscitation during initial management of RAAA before proximal aortic control is a predictor of increased postoperative mortality.88 Therefore, resuscitation that maintains the patient in a conscious state and prevents ST-segment depression is used to stabilize patients until definitive repair can be carried out.84 Currently, evidence regarding resuscitation with blood versus crystalloids is limited. If blood is available, it should accompany the patient during transfer and can be given instead of crystalloid solutions to maintain consciousness.
Operative Preparation
In the initial evaluation blood should be crossmatched so that it will be available at surgery. In the operating room, trained anesthesia, nursing, and technical personnel can prepare the patient for surgical operative or endovascular intervention. Large-bore access, insertion of an arterial line, and placement of a Foley catheter can be done simultaneously. The patient is prepared and draped awake before anesthesia and is induced with agents designed to have minimal effect on blood pressure. This allows the repair to be commenced immediately after induction. Alternatively, EVAR for RAAA can be carried out through a percutaneous approach, with local anesthesia and conscious sedation.
Operative Strategies: Endovascular Repair
EVAR is rapidly becoming the preferred method of therapy for RAAA, particularly for institutions that have the infrastructure for emergent EVAR. Data from the Nationwide Inpatient Sample reported that use of EVAR for RAAA has significantly increased over time, from 5.9% in 2001 to 18.9% in 2006.80 Data published on U.S. Medicare beneficiaries in 2012 indicated that by 2008, the proportion of RAAA treated by EVAR had increased to 31%.89 Furthermore, recent data indicate that at institutions with infrastructure for both EVAR and OSR for RAAA, by 2012, most RAAA patients (>70%) undergo EVAR (Fig. 133-4).8 Currently, OSR is generally reserved for those who are anatomically unsuitable for EVAR, for institutions that do not have the infrastructure to provide emergent EVAR, and when EVAR fails to seal the AAA.
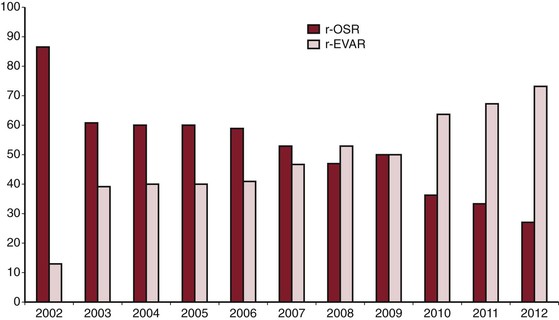
Figure 133-4 Ruptured abdominal aortic aneurysm treated by open surgical repair (r-OSR) versus endovascular repair (r-EVAR) at Albany Medical Center from 2002 to 2012.
Initial Management
A Standardized Protocol-Based Approach
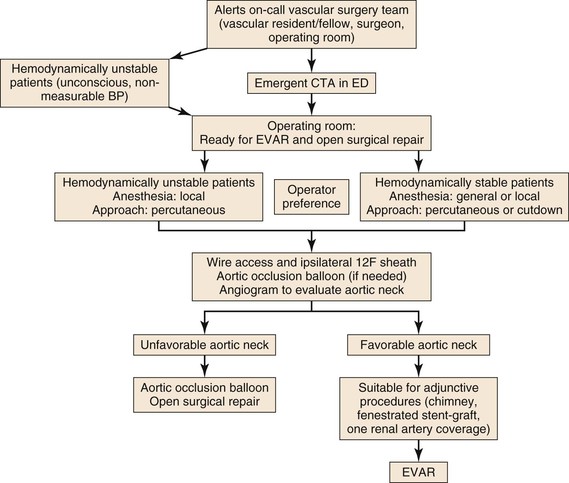
Introduction of EVAR for RAAA has forced us to re-evaluate protocols for patient transfer to the operating room for treatment. Today, the question is how to develop systems that allow patients with aneurysm rupture to undergo prompt endovascular repair. There are several obstacles to EVAR for all RAAA patients: anatomic suitability for EVAR, availability of dedicated staff and equipment to perform emergent EVAR at all hours, feasibility of treating hemodynamically stable and unstable patients by EVAR, and ability of the surgeon and interventionalists to manage unexpected scenarios under emergent circumstances. Many centers have developed a protocol that includes a heightened awareness among the emergency department staff to suspect the diagnosis of RAAA and instructions to notify the on-call vascular surgeon and the operating room staff.76,77,90 Hemodynamically stable patients undergo CT scan and are transferred to the operating room; hemodynamically unstable patients are directly transferred to the operating room without a preoperative CT scan for an endovascular-first approach and conversion to OSR as needed (Fig. 133-5).91 If necessary, thin-slice CT angiography with contrast enhancement can be accomplished with lower volumes of contrast agent than usual to assess neck diameter, angulation, and iliac size.92
Operating Room Setup
Not all RAAA patients can undergo endovascular repair. Therefore, all operating room and hybrid endovascular–operating room suites should be set up to facilitate EVAR as well as OSR. We have found it best to set up the room for endovascular repair with standard needles, wires, catheters, and sheaths open on a sterile table; to have the surgical instruments in the room, if needed; and, as the anesthesiology team prepares the patient, to set up the fluoroscopic equipment and supplies. Adequate resuscitation of patients with RAAA is vital to a successful outcome. As long as the patients maintain a measurable blood pressure, permissive hypotension can minimize ongoing hemorrhage.
Technique
Arterial Access
The patient is prepared and draped in a supine position. Using a percutaneous (preferred) or femoral artery cutdown (if required), ipsilateral access is obtained with a needle, floppy guide wire, and guiding catheter. The floppy guide wire is exchanged for a super-stiff wire. This is used to place a large sheath (12F to 14F × 45-cm length) in the ipsilateral femoral artery. The sheath is advanced up to the juxtarenal abdominal aorta so an aortic occlusion balloon can be deployed, if needed. A compliant occlusion balloon should always be available during emergent EVAR; in hemodynamically unstable patients, the occlusion balloon is advanced through the sheath over the super-stiff wire into the supraceliac abdominal aorta under fluoroscopic guidance, and the balloon is inflated as needed. Contralateral femoral access is obtained through a percutaneous or cutdown approach, and a marker flush catheter is advanced to the juxtarenal aorta for arteriography.
Stent-Graft Placement
The choice of stent-graft main body is based on aortoiliac morphology. In hemodynamically stable patients, after the initial arteriogram, the aortic occlusion balloon is removed from the ipsilateral side and the stent-graft main body advanced under fluoroscopic guidance. This limits the number of catheter exchanges. In hemodynamically unstable patients who require inflation of the aortic occlusion balloon, the marker flush catheter on the contralateral side is exchanged for the stent-graft main body, which is delivered up to the aortic neck. Arteriography is performed through the sheath that is used to support the aortic occlusion balloon, the tip of the stent-graft main body is aligned with the lowermost renal artery, the occlusion balloon is then deflated and withdrawn with the delivery sheath into the AAA, and the stent-graft main body is rapidly deployed. The remainder of the EVAR procedure is performed as in elective circumstances: the tip of the stent-graft main body is aligned with the lowermost renal artery, the contralateral gate is aligned to allow cannulation, and the ipsilateral and contralateral iliac extensions are planned and deployed as needed, as discussed in detail in Chapter 89.
Preoperative CT Scan
The hemodynamic status of the RAAA patient determines whether to obtain a preoperative CT scan or not. Most patients can tolerate a preoperative CT scan to evaluate the likelihood of EVAR and for stent-graft sizing. As discussed above, data on the feasibility of preoperative CT in patients with RAAA indicate that 88% of patients die more than 2 hours after admission, with the interval between hospital admission and death of 10.5 hours.93 This suggests most patients with RAAA have the time to undergo an emergent CT scan.
Anatomic Suitability for EVAR
The proportion of RAAA patients who are suitable for EVAR varied from 47% to 67% in two meta-analyses.94,95 Compared with AAAs, RAAAs have larger infrarenal aortic diameters and shorter neck lengths.96,97 These differences in AAA morphology affect the ability of EVAR to be performed. It is likely that institutions that treat a higher proportion of RAAAs by EVAR liberalize the indications for use in these high-risk patients.8
Several institutions have tried to identify the impact of EVAR on RAAA in patients with favorable versus unfavorable aortic neck morphology.94,95,98,99 We analyzed aortic neck morphology by CT scans in 180 consecutive patients with RAAA who underwent EVAR (74, 41%) or OSR (106, 59%).100 On the basis of EVAR device-specific favorable versus hostile aortic neck morphology, we identified that only 34% of patients with RAAA had neck morphology that would meet the indication for use for available stent-grafts. The EVAR patients with hostile aortic necks had a significantly higher incidence of female gender (32% vs. 19%; P < .01), mean maximum AAA diameter (7.4 vs. 5.5 cm; P < .01), abdominal compartment syndrome (20% vs. 4%; P < .01), type I endoleaks (16% vs. 4%), and need for all secondary interventions (77% vs. 40%; P < .01) during long-term follow-up. The 30-day mortality was the lowest in EVAR patients with favorable aortic necks and the highest in OSR patients (favorable, 8%; hostile, 23%; OSR, 43.4%; P < .01), and the EVAR patients with both favorable and hostile aortic necks had a better cumulative 3-year survival than OSR patients did (favorable, 64%; hostile, 67%; OSR, 44%; P < .01).
Choice of Anesthesia and Approach
Depending on operator and institutional experience, EVAR for rupture can be performed under local anesthesia through a percutaneous approach or with general anesthesia by femoral artery cutdown. One of the benefits of local anesthesia with conscious sedation is maintenance of “sympathetic tone” in the hemodynamically compromised patients. However, this should be done only by those who are comfortable obtaining percutaneous access and using closure devices. In hemodynamically unstable patients with weak or absent femoral pulses, this can be difficult but may be aided by use of ultrasound. The advantages of local anesthesia must be balanced by the potential difficulties as the patient might not be coherent and cooperative enough to lie still.76,77 To reduce operating time, particularly in hemodynamically unstable patients, some have suggested starting the procedure under local anesthesia, obtaining percutaneous access, positioning the aortic occlusion balloon as needed, performing EVAR, and then, after RAAA exclusion, inducing general anesthesia and removing femoral sheaths by femoral cutdown.91 Others have recommended a total percutaneous approach with local anesthesia and conscious sedation.101
Aortic Occlusion Balloon
The appropriate use of aortic occlusion balloons in hemodynamically unstable patients is vital to the success of EVAR. Access for aortic occlusion balloons can be obtained through the brachial or the femoral artery. There are several advantages of the femoral approach: it allows the anesthesia team to have access to both upper extremities for arterial and venous access; the patients who require the aortic occlusion balloon are often hypotensive, and in these patients, percutaneous brachial access can be more cumbersome and time-consuming than femoral cutdown; the currently available aortic occlusion balloons require at least a 12F to 14 F sheath, which requires a brachial artery cutdown and repair; and stiff wires and catheters across the aortic arch without prior imaging under emergent circumstances might lead to other arterial injuries or embolization, causing stroke. Occlusion balloons are composed of compliant materials such as polyurethane, latex, or silicone and have low burst pressures of less than 5 atm. Their primary function is not angioplasty but molding to the surrounding aorta with gentle inflation to occlude the proximal aorta.
Several important points should be considered during placement of an aortic occlusion balloon. The sheath supporting the balloon should be advanced and supported fully into the aortic neck before inflation of the balloon because this will prevent downward displacement and prolapse of the occlusion balloon into the aneurysm sac (Fig. 133-6).91 Inability to fully engage the sheath into the aortic neck due to significant aortoiliac stenosis, calcifications, or tortuosity might result in downward displacement of the inflated occlusion balloon; this often requires forward traction on the inflated balloon catheter to maintain adequate position at the suprarenal and supraceliac aorta (Fig. 133-7).91 If inflation of the aortic balloon is required to maintain a viable blood pressure, just before deployment of the stent-graft main body, the aortic occlusion balloon should be deflated from the suprarenal level and withdrawn. The stent-graft main body is subsequently deployed; this will avoid trapping the compliant aortic occlusion balloon between the aortic neck and the stent-graft. This temporary deflation of the aortic occlusion balloon rarely results in hemodynamic collapse and usually is of little consequence. In hemodynamically unstable patients, the occlusion balloon can be redirected into the aortic neck from the side ipsilateral to the stent-graft main body after the main body is deployed and reinflated at the infrarenal aortic neck within the stent-graft main body. This allows aortic occlusion and does not interfere with the remainder of the endovascular procedure (Fig. 133-8).91,102
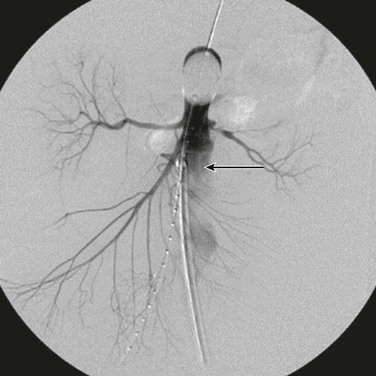
Figure 133-6 The sheath (arrow) supporting the aortic occlusion balloon should be advanced and supported fully into the aortic neck to prevent downward displacement and prolapse of the occlusion balloon into the abdominal aortic aneurysm.91
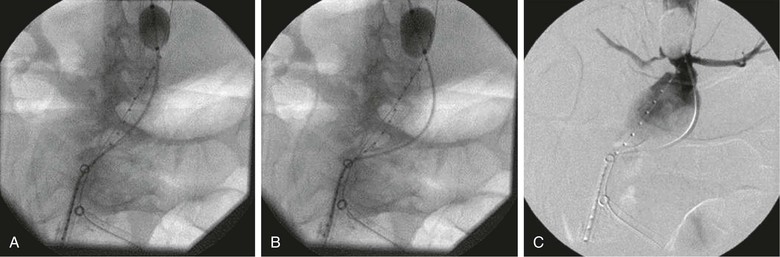
Figure 133-7 A-C, Inability to fully engage the sheath into the aortic neck due to significant aortoiliac stenosis, calcifications, or tortuosity might result in downward displacement of the inflated occlusion balloon. This often requires forward traction on the inflated balloon catheter to maintain adequate position at the suprarenal and supraceliac aorta.91
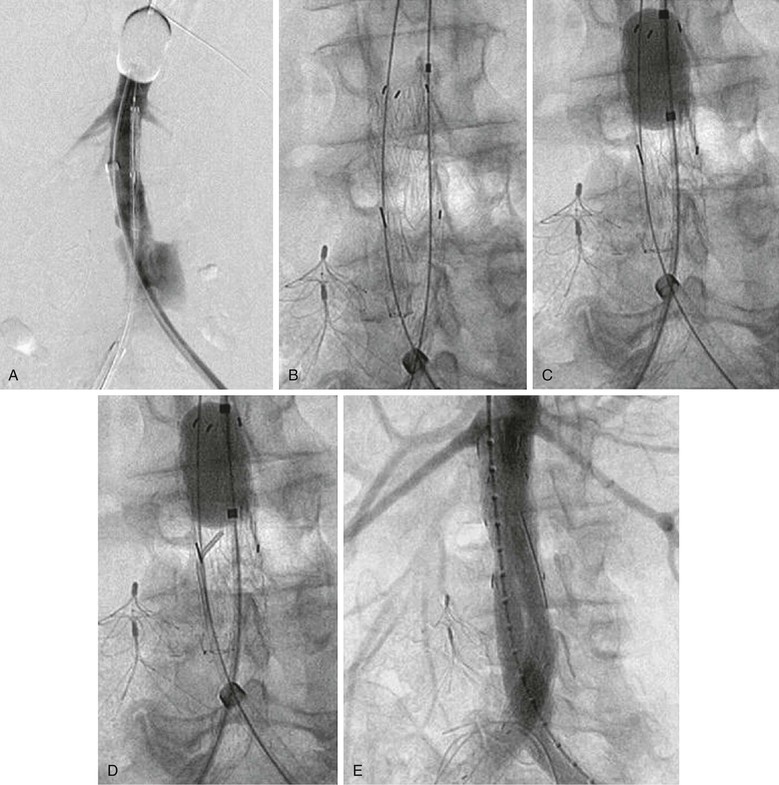
Figure 133-8 Managing the aortic occlusion balloon during stent-graft deployment. A, The inflated suprarenal aortic occlusion balloon is advanced through the left femoral approach, the stent-graft main body is advanced through the right femoral approach, and arteriography is performed through the left femoral sheath supporting the occlusion balloon. B, The aortic occlusion balloon is deflated and retracted from the aortic neck, and the stent-graft main body is subsequently deployed. This avoids trapping of the compliant aortic occlusion balloon between the aortic neck and the stent graft. C, In hemodynamically unstable patients, the occlusion balloon can be redirected into the aortic neck from the side ipsilateral to the stent-graft main body and reinflated at the infrarenal aortic neck within the stent-graft main body before contralateral gate cannulation. D, After the occlusion balloon is reinflated in the stent-graft main body in hemodynamically unstable patients, the contralateral stent-graft gate can be cannulated, and contralateral stent-graft extensions are placed as needed. E, After contralateral iliac extension and ruptured abdominal aortic aneurysm exclusion, the occlusion balloon can be removed, as shown in the completion arteriogram.91
Bifurcated versus Aortouniiliac Stent-Grafts for Ruptured Abdominal Aortic Aneurysm
Choice of stent-graft is determined by the patient’s aortoiliac morphology. Factors favoring the use of an aorto-uniiliac stent-graft for RAAA include inability to access the contralateral gate expeditiously and inability to access the contralateral iliac artery due to significant occlusive disease or tortuosity. Several studies have documented the use of aorto-uniiliac devices for EVAR of RAAA with outcomes similar to those with the use of bifurcated stent-grafts.103,104
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree