Chapter 132
Abdominal Aortic Aneurysms
Endovascular Treatment
Ronald M. Fairman, Grace J. Wang
Based on a chapter in the seventh edition by Timothy A.M. Chuter and Darren Schneider
Endovascular abdominal aortic aneurysm (AAA) repair was initially described by Volodos in 1986.1 Later, in 1991, Juan Parodi published his experience with retrograde deployment through the femoral arteries of a stent-anchored, Dacron-prosthetic graft that would act to depressurize the aneurysm sac and thus reduce the risk of aneurysm rupture.2 From there, the field of vascular surgery was radically transformed, and the era of endovascular aneurysm repair (EVAR) was born. This chapter focuses on the outcomes of EVAR and their comparison with open surgical repair outcomes. The technique of EVAR is discussed in detail in Chapter 90.
Graft Types
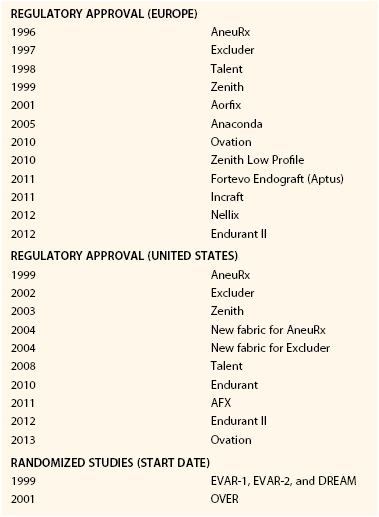
The initial grafts were constructed by suturing a balloon-expandable or self-expanding stent to a Dacron tube graft. These early grafts were tubular or “aorto-aortic” in design. In 1993, the unibody bifurcated aortobiiliac stent was developed3 and the modular bifurcated design quickly followed.4 Regulatory approval for the abdominal aortic stent graft occurred in 1996 in Europe, followed by the United States in 1999. Although the timeline (Table 132-1) suggests that the development and advancement of EVAR were part of a natural evolutionary process of technology, significant problems occurred along the way. The Guidant Ancure device (Indianapolis, Ind), which was approved in 1999 and was one of the first commercially approved devices, was recalled in 2001 because of delivery system failure in greater than 30% of procedures. The malfunction resulted in lodgment of the delivery system within the vasculature and inability to remove it without resorting to open surgery or unconventional means. Deaths were attributed to this delivery system failure, although the mortality was still less than reported following open repair.5 Follow-up of procedures using the Ancure device also revealed attachment site hook fractures and iliac limb stenosis and occlusions attributed to the graft limbs being unsupported by stents.6,7 Subsequent stent grafts have more often employed a fully supported main body and limbs. The original AneuRx graft (Medtronic, Minneapolis, Minn), although well received because of its ease of deployment and smaller profile, was plagued with a higher migration rate.8–10 Subsequent modifications and iterations have improved on early results, making the device less rigid and the fabric more robust. The Vanguard endograft (Boston Scientific, Natick, Mass) was prone to fabric tears caused by fatigue erosion of the stents against the fabric, resulting in fabric perforation.11 This observation appropriately influenced the design of future stent grafts and led to the secure suturing of stents to the fabric to prevent excessive motion over time. The Excluder graft (WL Gore, Flagstaff, Ariz) had a propensity for type IV endoleak due to the porosity of the fabric. Subsequent development of a low-porosity fabric resulted in an improvement in the rate of aneurysm sac shrinkage and marked reduction in the incidence of sac expansion.12 The Talent LPS graft exhibited stent spring fractures as well as longitudinal C-bar breaks. Chemical etching and medial placement of the longitudinal bar proved to prevent stent fatigue and reduced the overall failure rate.13 Since it has become commercially available, the Zenith stent graft has undergone few fundamental iterative changes, although it is now a more flexible design with delivery system enhancements. In fact, much of the long-term data available to date come from studies with a large representative of Zenith stent grafts (DREAM, EVAR-1, EUROSTAR).
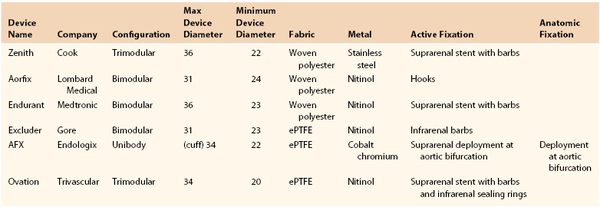
The modern version of stent graft design is a bifurcated graft, most commonly using a modular system to allow for the most flexibility with regard to patient anatomy. Currently, six FDA-approved infrarenal endografts are commercially available for the treatment of infrarenal AAAs (Fig. 132-1 and Table 132-2). Each device varies in terms of the type of fabric and stent composition, as well as method and anatomic location of fixation. Most stent graft designs have favored suprarenal stents (Zenith-Cook Medical, Bloomington, Ind; AFX-Endologix, Irvine, Calif; Endurant-Medtronic, Minneapolis, Minn; Talent-Medtronic, Minneapolis, Minn; Ovation-Trivascular, Santa Rosa, Calif) as a means to inhibit downward migration and development of type I endoleak and endograft failure. Additionally, the Zenith, Endurant, Excluder, and Ovation stents have barbs to provide active, rather than passive, fixation. Ovation stents have unique polymer-filled sealing rings to create an enhanced seal at the aortic neck and endolegs. The AFX device is the only one that touts passive fixation, whereby the flow divider of the stent graft sits on the aortic bifurcation.

Figure 132-1 A, Cook Zenith stent graft. B, Lombard Medical Aorfix stent graft. C, Medtronic Endurant stent graft. D, Gore Excluder stent graft. E, AFX(®) Endovascular AAA System (Image courtesy of Endologix, Inc.). F, Trivascular Ovation stent graft. (Image © TriVascular, Inc. Used with permission.).
Randomized Trials of Evar Versus Open Repair
Perioperative Outcome
EVAR-1 was a randomized prospective UK study that compared open abdominal aortic aneurysm repair with EVAR in patients who were fit enough to undergo open surgical repair.14 From 1999 to 2003, 1082 patients were randomized to either the EVAR or open aneurysm repair treatment arm. The 30-day mortality rate was reduced in the EVAR group (1.7% versus 4.7%), although secondary interventions were more common in the EVAR group (9.8% versus 5.8%). The DREAM trial, which took place in the Netherlands, was a multicenter randomized trial (enrolling from 2000 to 2003) that compared open repair to EVAR in 345 patients.15 It similarly demonstrated a reduction in operative mortality in EVAR patients (1.2% versus 4.6%). The combined rate of operative complications and mortality also favored EVAR (4.7% versus 9.8%), with the majority of complications accounted for by pulmonary issues. The OVER trial was a randomized study that enrolled 881 patients from 42 VA centers to undergo either EVAR or open repair.16 Perioperative mortality was improved in the EVAR group (0.5% versus 3.0%), yet no difference was seen in mortality at 2 years (7.0% versus 9.8%). The Anevrisme de l’aorte abdominal: Chirurgie versus Endoprosthese trial compared EVAR with open surgical repair in low- to moderate-risk patients. In contrast to the previously mentioned trials, in-hospital mortality and the incidence of postoperative complications were not statistically different. In addition, with a median follow-up of 3 years, no difference was observed in survival or incidence of major events.17 Although not conclusive, the results from these trials indicate the need to better define the unique risks associated with each procedure: more physiologic stress with the large index procedure of open surgery with its attendant complications versus the need for continued surveillance, possible need for future endovascular reintervention, and a small but persistent risk of rupture with EVAR.
Long-Term Outcome
Although much literature is available on the long-term performance of first- and second-generation aortic stent grafts,18–23 some of which are no longer on the market, findings are incomplete on the long-term behavior of current stent grafts. A comparison between current stent grafts and those that are off the market reveals a striking difference in regard to rates of migration, rupture, conversion, and reintervention.18,24
Survival analysis from EVAR-1 demonstrated no difference in all-cause mortality at 6 years, although only 24% of patients survived until the end of the study period. The initial survival benefit of EVAR was lost within 2 years of repair, secondary to a higher death rate from cardiovascular causes among those patients who had undergone EVAR.25 The rate of graft related complications and need for reinterventions was significant at 4 years, likely accounting for the higher aneurysm-related death rate in the EVAR group. The rates of aneurysm-related death converged at 6 years in these two groups. The 2-year follow-up study from the DREAM trial showed similar results with a reduction in aneurysm-related mortality in the EVAR group compared with the open surgery group (2.1% versus 5.7%), which was not reflected in overall mortality.26 Mortality from cardiovascular causes in the EVAR group again contributed to the equalization of survival between groups at 2 years. As expected, the need for reintervention was higher in the EVAR group and was three times that of the open group in the first 9 months after randomization. With publication of results from the 6-year follow-up, secondary interventions were again higher in the EVAR group: 30% versus 19.1%. The cumulative 6-year survival in the two groups was similar, at 69.9% for the open group and 68.9% for the EVAR group.27 The OVER trial 2-year results revealed that although the perioperative advantage of EVAR was still realized at 3 years, survival was similar between groups beyond this time period.28
Although the results from EVAR-1, DREAM, and the OVER trial rather conclusively demonstrate the improved perioperative morbidity and mortality profile of EVAR compared with open aortic aneurysm repair, concerns remain over the long-term durability and survival benefit of EVAR. Earlier experience with EVAR showed how device failure could lead to migration, type I endoleak, and aneurysm rupture. Although device modifications and improvements in design have led to a decrease in device failure, the 6-year follow-up data from the UK EVAR-1 trial indicated no differences in overall or aneurysm-related mortality compared with open repair in the long term. Furthermore, EVAR was associated with a higher rate of interventions and was more costly.25 Data from large registries such as EUROSTAR have estimated a reintervention rate of 5% per year and a continued rupture rate of 1% per year despite EVAR.29,30 Further long-term follow-up will help elucidate these issues. As the technology continues to evolve and operator sophistication continues to grow, one needs to keep in mind that the success of EVAR is highly dependent on the patient’s anatomic suitability for the procedure. Such factors that determine anatomic suitability include neck diameter, neck length, and angulation. In EVAR-1, 54% of patients were considered to be anatomically suitable for EVAR, but this percentage ranged from 6% to 100% across institutions, underscoring the variation in the assessment of this variable. More cohesiveness in the determination of anatomic suitability will improve outcomes following EVAR.
Population-Based Results
In general, institutional series results are reported by academic centers with a high level of expertise. Even controlled, prospective, multicenter studies reflect the beneficial effects of rigorous patient and investigator selection. Thus, a more accurate picture can be gleaned from a large, well-defined, but unselected population. Statewide and nationwide audits fill this role well, although procedure-specific information may be sparse because the study is usually based on administrative claims. Studies of this kind have confirmed the short-term advantages of EVAR over open AAA repair.31,32 One study, an analysis of Medicare data from 2001 to 2004, was a population-based study examining the outcomes of Medicare beneficiaries who underwent EVAR or open repair.33 One can assume that most of the repairs in this study were performed with AneuRx stent-grafts because little else was available until 2003 and the transition to other devices was a slow process. The overall perioperative mortality rate was 1.2% for EVAR and 4.8% for propensity score–matched surgical controls. While perioperative mortality was lower with EVAR, late survival was similar between the two groups, although the survival curves did not converge until after 3 years. By 4 years, the EVAR group had more aneurysm ruptures as well as a higher reintervention rate. In contrast, a higher rate of laparotomy-associated complications was seen in the open repair group. This was further examined in a study of late survival with reinterventions and readmission after open and endovascular aneurysm repair.34 Reintervention and readmissions were more frequent after EVAR than after open repair (7.56 versus 6.96/100 person-years), but the majority of these reinterventions were minor endovascular interventions. Notably, rupture was five times more common after EVAR compared with open repair, but with a relatively low rate overall. In the open repair group, as expected, laparotomy-associated reinterventions were more common and were associated with a mortality rate of 12.2%. The study concluded that survival was negatively impacted by reintervention or readmission after EVAR or open surgery, which contributed to the loss of survival benefit of EVAR over time. Notably, the late failures of EVAR are consistent with the known failure modes of the AneuRx stent-graft, which was in widespread use throughout the United States at the time of the study.20,35 In contrast, a more recent study of Medicare beneficiaries using the Medicare Standard Analytic file, which included patients from 2003 to 2007, showed that open repair was associated with an increased risk of all-cause mortality and AAA-related mortality over a follow-up period of 5.7 years.36 The initial 30-day mortality associated with open repair was sustained during the entire follow-up period.
Meta-Analysis
A large segment of the available data on EVAR, consisting of 161 papers and 278,862 patients, was examined in a recent meta-analysis.37 The relatively high overall mortality rate (3.3%) in the combined series reflects the influence of poor early results. These authors used a technique of meta-regression to weigh the effects of different studies over time and assess temporal changes in outcome. The data show striking heterogeneity, some of which is attributable to the effects of changing technique and device design. Both mortality rate and endoleak rate showed steady improvement between 1992 and 2002. Based on the regression line, the perioperative mortality rate was estimated to have fallen to approximately 1.4% in 2002, which is very close to the rates seen in the Lifeline, EVAR-1, and DREAM studies (see earlier discussion).
The data from these population-based studies suggest that as technology and practitioners continue to improve, the benefit of EVAR over open repair in long-term survival will be apparent.
EVAR Compared with Medical Management
The question of whether EVAR is better than medical management in high-risk patients was addressed by the EVAR-2 trial, in which 338 patients who were unfit for an open repair were randomized to either EVAR or medical management.38 The 30-day mortality rate for EVAR was 9%, although 3.6% of these deaths were from rupture while awaiting EVAR, since the median time to intervention was 57 days. Further, 25% of patients assigned to medical management underwent EVAR either because of patient preference or surgeon preference, with a strikingly low mortality rate. Given the number of AAA ruptures in the EVAR group while awaiting surgery, as well as crossover of the medically treated patients to the treatment group, it is perhaps not surprising no difference was seen in aneurysm-related or overall mortality between groups at 4 years. Despite its shortcomings, EVAR-2 correctly underscores the fact that very high-risk patients do not benefit from AAA repair, because they die from other causes before a benefit can be realized. However, it appears that EVAR can be performed safely by experienced surgeons in carefully selected high-risk patients.
Other studies provide data on performing EVAR in high-risk patients.39–41 Data from the Department of Veteran Affairs (VA) National Surgical Quality Improvement Program on high-risk veterans undergoing EVAR showed a 30-day mortality rate of 3.4% and a 1-year mortality rate of 9.5%, which was significantly reduced compared with open repair (5.2% and 12.4%, respectively).41 A subset analysis of high-risk patients from five industry-sponsored Food and Drug Administration trials showed a perioperative mortality rate of 2.9%. Both studies had open surgical controls, which suggests that the relatively improved survival percentages may have been due to a “healthier” high-risk cohort. The VA large aneurysm study, which was an observational study of unfit patients, showed a 1-year rupture rate of 9.4% for aneurysms measuring 5.5 to 5.9 cm, 10.2% for aneurysms 6.0 to 6.9 cm, and 32.5% for aneurysms measuring 7.0 cm or larger.42 Thus very large AAAs even in high-risk patients may benefit from EVAR, but often such AAAs are not anatomically suited for EVAR, so careful patient selection is critical.
Comparison of Different Stent Grafts
There is a dearth of data to allow appropriate comparison among different EVAR devices, due to lack of large numbers and lack of long-term follow-up. From the EUROSTAR data,40 the AneuRx and Talent stent grafts had the highest rates or migration, endoleak (types I and III), and conversion. The Zenith stent graft, while having the highest rate of aneurysm sac shrinkage and lowest rate of migration, had the highest rates of limb occlusion. The Excluder stent graft had the lowest rate of limb occlusion. There are limitations to the EUROSTAR Registry—including voluntary, not mandatory, enrollment—which likely introduce bias. The Lifeline Registry consists of data from patients collected under four multiinstitutional investigational device exemption clinical trials for FDA approval with mandatory 5 year follow-up.43 Unfortunately, the only device-specific information that could be obtained was for the Ancure device, which is no longer commercially available. No data are available on Zenith stent grafts. Resch et al devised a method to evaluate the amount of longitudinal traction required to disrupt endograft fixation to cadaveric aortas, with a sutured anastomosis used as a comparison.44 Although a force of 150 Newtons was needed to disrupt a surgical anastomosis, all endografts required much less traction for dislodgment. The Zenith required the most force to be exerted (24 N), followed by the Ancure (12.5 N), Vanguard (9.0 N), and Talent (4.5 N). A similar study by Malina et al showed that barbs and hooks increased the fixation 10-fold, whereas radial force had no impact.45 Although one can review the corresponding industry-sponsored studies, differences in study design, patient populations, follow-up, and definitions make comparison challenging. Overall, for stent grafts with long-term data available, the migration rate appears to be lowest for stent grafts that offer proximal active fixation.
Complications of Endovascular Aneurysm Repair
Endoleak
An endoleak is defined as persistent blood flow in the aneurysm sac following stent grafting. Pressurization of the aneurysm sac can then lead to aneurysm rupture post-EVAR. Endoleaks are categorized into five different types, which differ in etiology as well as treatment (Fig. 132-2). The five types are discussed in the following sections.46–52

Figure 132-2 A type I endoleak (periprosthetic) occurs at the proximal or distal attachment zones (or at both). A type II endoleak is caused by retrograde flow from patent lumbar or inferior mesenteric arteries. A type III endoleak arises from a defect in the graft fabric, an inadequate seal, or disconnection of modular graft components. A type IV endoleak is due to graft fabric porosity, which often results in a generalized mild blush of contrast material within the aneurysm sac. (From White GH, et al: Type III and type IV endoleak: toward a complete definition of blood flow in the sac after endoluminal AAA repair. J Endovasc Surg 5:305-309, 1998.)
Type I
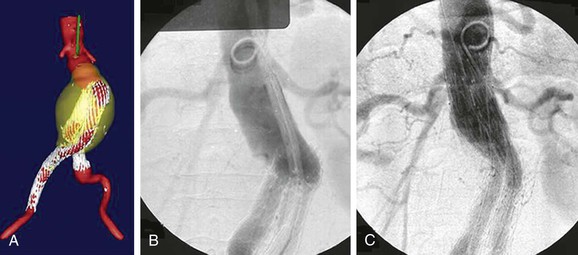
Type I endoleak is defined as persistent blood flow into the sac either from around the graft proximally (type IA) or distally (type IB). When recognized in the operating room, type I endoleaks are typically addressed by performing proximal device extension and/or bare metal stent deployment to buttress radial support in the neck, with or without renal artery snorkel/encroachment techniques to preserve renal artery blood flow.53 The Aptus EndoStapling System (Heli-FX, Aortic Securement System, Sunnyvale, Calif), which uses screws to secure the stent graft to the aortic wall, may also be used to enhance the proximal seal. If the main body or trunk of the prior stent graft is too short for the deployment of a proximal extension, an aortouniiliac device may be employed necessitating a femoral-femoral artery bypass and embolization of the contralateral proximal iliac artery with an occlusion device. The patency rate of femoral to femoral artery bypass done with aortouniiliac stent grafting for treatment of aortic aneurysmal disease is high, with a primary patency at 54 months of 90.9% and assisted primary and secondary patency rates of 97.7% and 100% at 66 months.54 When type I endoleak is detected late, it is typically secondary to caudad migration of the stent graft or continued dilatation of the neck. Provided that there is a commercial device available to seal the dilatated aortic neck, a proximal extension may be deployed to achieve a proximal seal (Fig. 132-3). If type I endoleak continues after proximal stent graft extension or bare metal stent placement, embolization with glue and or coils has been shown to be a useful adjunct in treating persistent type I endoleak in a small series.55 A fenestrated graft may also be employed to extend the seal zone to the juxta- and suprarenal aorta (this is discussed in detail in Chapters 91 and 137). Distal common iliac artery dilatation with type IB endoleak is retreated with hypogastric artery occlusion and extension to the external iliac artery or by use of the Zenith branched iliac device. Another described maneuver to treat proximal type I endoleak includes the aortic wrap method with a 12-mm Hemashield patch secured around the aortic neck.56 If the previously described maneuvers are not successful in fixing the endoleak, operative explant of the stent graft is necessary because of the high risk of rupture associated with type I endoleak.
Type II
Type II endoleak is defined as persistent sac filling from backbleeding side branches (i.e., the inferior mesenteric artery, lumbar arteries, middle sacral artery). Postoperative CT scans show type II endoleak in 10% to 20% of patients following EVAR.46–48,57 In comparison with type I and type III endoleaks, type II endoleaks have a relatively benign course, with as many as 80% resolving spontaneously within 6 to 12 months of stent graft repair.18 Furthermore, the risk of aneurysm rupture due to a type II endoleak is small. Multivariate analysis of the EUROSTAR data showed no association between type II endoleak and risk of aneurysm rupture or need for surgical conversion.48 A recent meta-analysis likewise demonstrated the rarity of aneurysm rupture with isolated type II endoleak.58 It is for this reason that type II endoleaks are not treated unless aneurysm sac enlargement is documented. Persistent type II endoleaks are usually associated with a large endoleak cavity,59 flow between inflow and outflow arteries,60 and large patent inferior mesenteric and lumbar arteries.61 Embolization can be performed using coils or N-butyl cyanoacrylate glue. The transarterial approach can be undertaken to embolize the inferior mesenteric artery through the superior mesenteric artery from the marginal artery of Drummond. Another technique involves catheterizing the sac via the femoral artery by advancing the wire and catheter in between the iliac limb and the iliac artery into the abdominal aortic aneurysm.62 The translumbar approach uses a direct aneurysm sac puncture technique (Fig. 132-4). Regardless of the approach, type II endoleaks physiologically behave like A-V malformations, and embolization of the nidus of the endoleak, including the inflow and outflow vessels, is the optimal approach. Retroperitoneal endoscopic ligation of the lumbar arteries has also been described.63 If the previous interventions fail to be successful, operative explant or direct ligation of the backbleeding vessel is required.
Type III
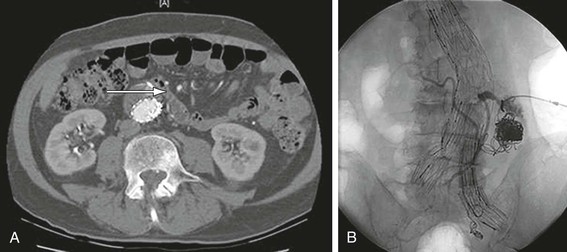
A type III endoleak results from fabric erosion or a leak between stent graft components. Only modular grafts are at risk for component separation, but all devices are at risk for graft failure. Rates of type III endoleak range from 0% to 1.5%.48,64 Type III endoleak from fabric erosion is effectively treated with stent graft re-lining or bridging of components to effectively seal the hole (Fig. 132-5). Component separation is likewise treated with re-lining. In cases in which the device pieces are particularly separated in space due to excessive tortuosity, obtaining wire access through both components may be challenging, and transbrachial access with a snare may be useful in this situation. Plain radiographs are often more sensitive for detecting impending component separation because they offer a more global overview of the framework of the stent graft. While in the operating room, arteriography with the pigtail catheter in the main body of the stent graft and below the proximal fixation site can help to distinguish a type I from a type III endoleak.
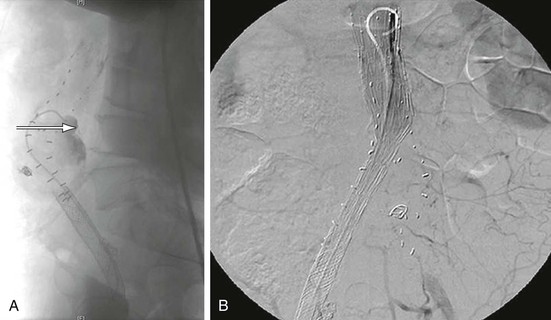
Figure 132-5 A, A patient who had undergone an EVAR 4 years previously with the Ancure device. His left limb was chronically occluded, which was causing claudication symptoms. CT scan additionally revealed continued aneurysm sac enlargement. A lateral projection arteriogram demonstrated erosion of the stent graft with contrast extravasation into the sac in the posterior portion of its main body (arrow). B, Aortouniiliac stent graft was deployed to re-line the original repair followed by femoral-to-femoral artery bypass to revascularize the contralateral limb to eliminate the type III endoleak.
Type IV
Type IV endoleaks are related to porosity of the graft fabric, occur less frequently with current-generation stent grafts, and are noted within 30 days of graft implantation. Type IV endoleaks are most commonly observed as a blush of contrast coming through the fabric of the stent graft on the completion arteriogram prior to heparin reversal. They usually resolve once graft interstices thrombose. No treatment is usually required because they are short-lived and resolve on their own. High graft porosity, as demonstrated by the earlier-generation Excluder graft, however, can lead to sac growth in the absence of demonstrable endoleak.12
Type V
Type V endoleak, or “endotension,” is defined as elevated sac aneurysm pressure without a demonstrable endoleak. It is generally believed that the etiology is an undetected endoleak or transmission of systemic pressure through thrombus. Endotension is typically detected as continued sac enlargement on CT scan but can also be detected by direct aneurysm sac pressure sensors (Cardiomems, Atlanta, Ga). Re-lining with proximal and/or distal extension may be initially performed. If this is not successful, operative explant is necessary.
Endoleak Detection
Contrast-enhanced CT scan is the gold standard for detection of endoleaks. A complete study consists of unenhanced, early, and delayed phases. The unenhanced scan shows mural calcium, which can be distinguished from contrast on subsequent series. Types I and III endoleaks are detected on arterial phase images whereas type II endoleaks are detected on delayed phase images. The location of the endoleak may give an indicator as to the origin of the endoleak. For example, a ventrally oriented leak is likely to be emanating from the inferior mesenteric artery, whereas a dorsally oriented endoleak is more likely to supplied by the iliolumbar arteries.50 If the endoleak is not demonstrated on three dimensionally reconstructed CT images, arteriography is warranted. Alternative imaging modalities include magnetic resonance imaging (MRI)65,66 and duplex ultrasound.67–69 MRI is an expensive imaging modality, and caution must be used with stainless-steel devices such as the Cook Zenith stent grafts.70 In addition, the earlier proposed benefit of use in patients with renal insufficiency has been overturned in light of the increased incidence of nephrogenic systemic fibrosis with gadolinium exposure in patients with a glomerular filtration rate less than 30.71 Duplex ultrasound does not require contrast administration and is not associated with radiation exposure, but its success depends greatly on the technologist, and it can be complicated by patient factors such as morbid obesity or presence of bowel gas. A recent meta-analysis revealed a pooled sensitivity of 0.83 and a pooled specificity of 1.00 for duplex ultrasound detection of type I and type III endoleaks.72 In patients with renal insufficiency in which contrast-induced nephropathy is a concern, duplex ultrasound can offer a good surrogate, providing information about sac size as well as presence and type of endoleak. Data from a relatively large study revealed that although duplex ultrasound yielded only a sensitivity of 67% compared with CT scan, no type I endoleaks or endoleaks requiring intervention were missed.68
Migration
Migration refers to caudal movement of the stent graft, which may lead to loss of proximal fixation and result in type IA endoleak. Cephalad migration of iliac limbs occurs less frequently, and rarely, stent graft may migrate proximally to encroach on the renal arteries. The tendency toward caudal migration increases with angulation of the neck,73 a short neck, neck thrombus, and large diameter of the neck.74 In the multicenter AneuRx trial, 8.4% patients experienced migration of their stent grafts, with low initial deployment of the device and short fixation length found to be significant predictors.75 Postprocedural causes for stent graft migration include aortic neck dilatation, sac shrinkage with concomitant sac shortening, and displacement of the stent graft from external compression.56,76,77 The most important planning strategy is adherence to the instructions for use, as much as possible. Preprocedurally, a fixation length of 15 mm is typically recommended, although some data suggest that as little as 10 mm may be effective in providing a seal, at least in the short term.78,79 In addition, avoiding angulation greater than 60 degrees between the infrarenal neck and the longitudinal axis of the aortic aneurysm is associated with less migration. In a particularly angulated patient, a more flexible stent graft should be chosen. The Aorfix stent graft (Lombard Medical Technologies, Tempe, Ariz) is the only stent graft approved for neck angulation greater than 60 degrees but still less than 90 degrees. In a flow model study comparing the Aorfix stent graft with other manufactured stent grafts of less flexible design, the endoleak rate in angulated necks was lower in the Aorfix-treated patients.80 Midterm results from a European multicenter study on the Aorfix also revealed acceptable results, although longer-term follow-up is needed.81
To avoid migration in patients with short and angulated necks, several manufacturers have modified their original devices to make them more flexible. In addition, the Zenith and the Excluder also offer active fixation with the use of barbs, which are an integral part of the device and become embedded in the aortic wall. The AFX stent graft (Endologix, Irvine, Calif) provides passive fixation via its “anatomic” seating on the aortic bifurcation. It is designed to allow for aortic extensions to be added proximally, which is ideal in the presence of a highly angulated neck. The Aorfix stent graft has proximal clips for active fixation of the device to the aortic wall. Its fish-mouth design also enhances the length of seal available at the neck. In addition, because of its unique ring stent design, it is very flexible and resistant to kink. The Aptus EndoStapling System helps to fix the proximal aspect of the main body to the aortic wall. The Anaconda (Vascutek, TERUMO Cardiovascular Systems Corp, Ann Arbor, Mich) is another device undergoing clinical trials in the United States. In addition to its unique flexibility, the Anaconda allows for repositioning after graft deployment, allowing for optimum graft placement.82 Treatment of migration is discussed in the earlier section on type I endoleak.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree