AAA with IVC Fistula
KOTA YAMAMOTO, TETSURO MIYATA, and ALAN DARDIK
Presentation
An 82-year-old man presents with dyspnea that started suddenly several months prior to the visit. He has a history of coronary intervention after myocardial infarction 6 years ago. On abdominal examination, a pulsatile mass is noted with continuous murmur. He has no history of abdominal or back pain. Edema and varicose veins of the left lower extremity are seen, but distal pulses are palpable. Plain chest x-ray shows pulmonary vascular congestion, cardiomegaly, and a small right pleural effusion. His EKG reveals P pulmonale and left ventricular hypertrophy.
Differential Diagnosis
There are three conditions to consider. Each of them is fairly simple to diagnose, but the problem with this patient is to evaluate if they are independent or related conditions. If related, then what is the pathophysiology?
1. Congestive heart failure
The dyspnea with x-ray and EKG findings show that this patient has congestive heart failure. Further workup is needed to evaluate its severity, as well as its etiology. Previously undiagnosed congenital abnormalities, such as patent foramen ovale, or central malignancies may contribute to the circulatory overload and heart failure.
2. Abdominal aortic aneurysm (AAA)
Pulsatile abdominal mass is a straightforward clue to the presence of an AAA. Workup is needed to differentiate AAA from an abdominal mass, such as a tumor. In addition, aneurysm size and shape need to be evaluated to plan treatment strategies.
3. Lower limb venous congestion
Lower limb edema accompanied with varicose veins is suggestive of venous congestion. The finding that the edema is unilateral, without any manifestations on the contralateral limb, suggests that the edema may be secondary to mechanical obstruction, such as deep venous thrombosis. Although edema may not be life threatening, further workup is needed to determine its etiology.
Workup
This patient undergoes contrast-enhanced CT scan of the abdomen (Fig. 1). This study shows an aneurysm involving the left common and internal iliac artery. In addition, contrast is seen in the inferior vena cava (IVC) (venous enhancement in the arterial phase), demonstrating the presence of a fistula between the artery and the vein (arteriovenous fistula [AVF]). The exact location of the AVF may be difficult to locate with the CT scan, but the continuous murmur located at the abdominal mass suggests that the AVF is probably located at the aneurysm.
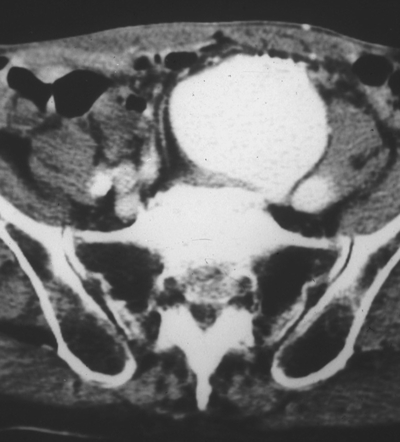
FIGURE 1 Contrast-enhanced CT. The vein (left-sided IVC) is enhanced at the same time as the iliac aneurysm.
Although not always necessary, angiograms can also be performed to evaluate the fistula (Figs. 2 and 3) The angiogram shows that this patient has a duplicated IVC, and the flow through the fistula goes directly into the left IVC. Angiogram of the left lower limb shows that the flow from the AVF refluxes retrogradely into the left lower limb veins.
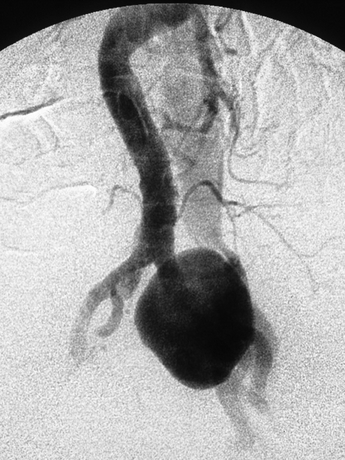
FIGURE 2 Angiogram showing the iliac aneurysm. A left-sided IVC is also detected as soon as the iliac aneurysm is seen.
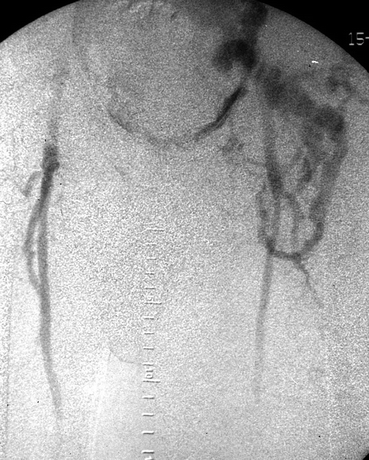
FIGURE 3 Angiogram of the lower limb. Left iliac and femoral veins can be seen as the distal retrograde flow below the fistula.
As preparation for surgery, systemic workup is necessary. Cardiac evaluation is especially necessary not only to understand the degree of heart failure but also to anticipate and prepare for potential physiologic changes that may occur after closure of the AVF.
Diagnosis and Treatment
Based on the information obtained from the CT scan and the angiogram, the primary diagnosis is the left common iliac artery aneurysm extending into the internal iliac artery. The aneurysm is likely to have ruptured into the left iliac vein forming an AVF between the iliac arteries and veins. This enormous flow through the AVF led to the precardiac overload upstream of the AVF and congestion of the left lower limb veins downstream of the AVF.
Aneurysmectomy accompanied with AVF closure is the best treatment that is a durable solution for all the pathophysiology. Recent advances in vascular surgery allow us to choose between two treatments for abdominal aortic aneurysmal repair.
One treatment option is endovascular aneurysmal repair (EVAR) using stent grafts. For EVAR in this patient, all the branches of the internal iliac artery would need to be embolized using coils and then the stent graft placed to cover the inflow into the internal iliac artery. After placement of the stent graft, the presence of an endoleak would suggest persistence of the fistula that should be considered for additional treatment; placement of a covered stent inside the caval side of the fistula may be a potential solution. However, prophylactic placement of a venous covered stent prior to aneurysm repair could potentially induce aneurysm rupture.
The other treatment option is open iliac artery aneurysm repair. In the open procedure, direct AVF closure can be performed in addition to aneurysmectomy with a graft replacement. The superiority between EVAR and open has been debated; surgeons are advised to take into account the benefits and risks of each procedure for individual patients.
Surgical Approach
Open repair of the iliac aneurysm with AVF closure is chosen for this patient. EVAR is not chosen to prevent the possibility that if the branches of the internal iliac artery were imperfectly occluded, residual type 2 endoleak could drain through the AVF, continuing potential for precardiac fluid overload and residual congestive heart failure. Open repair affords the ability for direct AVF closure but has the risk of performing open surgery on an octogenarian.
Under general anesthesia, a midline laparotomy is followed by full exploration of the abdomen. The bowels are set aside to the right side revealing the aorta and the iliac arteries. Inspection of the aneurysm leads us to decide that the terminal aorta and the right common iliac artery will be the proximal clamping sites. As for the distal clamping sites, the left external artery is soft, but the aneurysm precludes assessment of the internal iliac artery and will need to be occluded with a balloon catheter after opening the aneurysm. We also decide not to reconstruct the internal iliac artery. Arteries are mobilized and controlled; prior to clamping, a pneumatic tourniquet is applied to the left lower limb to decrease the venous inflow.
Opening the aneurysm reveals an AVF (25 × 10 mm) with tremendous back bleeding of venous blood. Immediately, large Foley balloon catheters are inserted through the AVF to control the bleeding from the common, external and internal iliac veins (Fig. 4). This internal iliac vein catheter is also used to occlude the main trunk of the internal iliac artery. Another option to control back bleeding is the use of two sponge-sticks, controlling the venous flow above and below and allowing oversewing of the fistula.
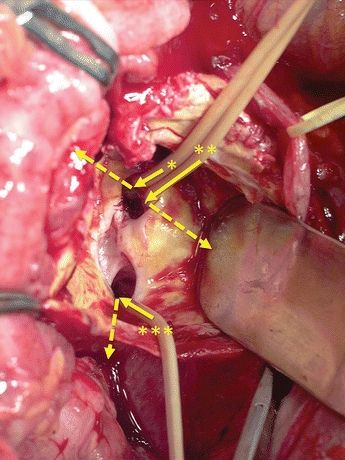
FIGURE 4 The AVF is confirmed after opening of the aneurysm. Through this fistula, three balloon catheters are inserted to occlude the iliac veins. The dashed arrow shows the direction of the veins that each catheter is placed. (*, Left common iliac vein; **, left external iliac vein; ***, left internal iliac vein.)



