Risk stratification for heart failure (HF) in patients with atrial fibrillation (AF) has not been well established. The aim of this study was to identify the predictors of HF events in patients with AF, consequently developing a new risk-scoring system that stratifies the risk for HF events. In this prospective, single hospital-based cohort, all patients who presented from July 2004 to March 2010 were registered (Shinken Database 2004–2009). Follow-up was maintained by being linked to the medical records or by sending study documents of prognosis. Of the 13,228 patients in the Shinken Database 2004–2009, 1,942 patients with AF were identified. Of the patients with AF, HF events (hospitalization or death from HF) occurred in 147 patients (7.6%) during a mean follow-up period of 776 ± 623 days. After identifying the parameters that were independently associated with the incidence of HF events (coexistence of organic heart diseases, anemia [hemoglobin level <11 g/dl], renal dysfunction [estimated glomerular filtration rate <60 ml/min/m 2 ], diabetes mellitus, and the use of diuretics), a new scoring system was developed, the H 2 ARDD score (heart diseases = 2 points, anemia = 1 point, renal dysfunction = 1 point, diabetes = 1 point, and diuretic use = 1 point; range 0 to 6 points). This scoring system discriminated the low- and high-risk populations well (incidence in patients scoring 0 and 6 points of 0.2% and 40.8% per patient-year, respectively) and showed high predictive ability (area under the curve 0.840, 95% confidence interval 0.803 to 0.876). In conclusion, the new H 2 ARDD score may help identify the population of patients with AF at high risk for HF events.
Atrial fibrillation (AF) is the most common arrhythmia among the developed countries, and its prevalence almost doubles with each decade of life. Along with stroke, heart failure (HF), which is a significant complication of AF, has been identified as an independent predictor for mortality in patients with AF. Our previous data suggested that HF remains an important target for treatment to improve the prognosis of patients with AF, indicating that a particular population at high risk for HF still exists among patients with AF. However, risk factors for HF in patients with AF have not been clearly identified. In the present study, we aimed to identify the predictors of HF events in patients with AF, whereby we subsequently developed a new risk-scoring system that stratifies risk for the incidence of HF events.
Methods
The Shinken Database was established for all new patients who visited The Cardiovascular Institute in Tokyo, Japan (Shinken is an abbreviated name in Japanese for the name of the hospital), and it excluded patients with active cancer and foreign travelers. The principal aim of this hospital-based database is to survey the prevalence and prognosis of cardiovascular diseases in patients in urban areas of Japan. The registry was started in June 2004, and thereafter, patients have been continually registered in the database. The data in the present study were derived from this database from June 2004 to March 2010 (Shinken Database 2004–2009), including 13,228 newly visiting patients, among whom AF was diagnosed in 1,942.
For each patient, an electrocardiogram and chest x-ray were obtained, and the patient’s cardiovascular status was evaluated using echocardiography, an exercise test, 24-hour Holter recordings, and blood laboratory data within 3 months after the initial visit, according to the decision of the attending physician. Information regarding the patient’s medications was obtained from the hospital database within 3 months after the initial visit. Details have been published elsewhere. The health status and the incidence of cardiovascular events and mortality were maintained in the database by being linked to the medical records of the hospital or by sending study documents of prognosis once per year to those who were referred to other hospitals. In the present data analysis, follow-up data that were collected after April 1, 2010, were excluded. Therefore, the end of the follow-up period of each patient was defined as 1 of the following: (1) the date of death, if the date was before March 31, 2010; (2) the final hospital visit or final response to our study documents of prognosis with confirmation that the patient was alive before March 31, 2010; and (3) March 31, 2010, when the date of death, the final hospital visit, or the final response to our study documents of prognosis was later than April 1, 2010.
The ethics committee of The Cardiovascular Institute granted ethical permission for this study, and all the patients gave written informed consent. The study was performed in accordance with the Declaration of Helsinki.
In the present study, AF was diagnosed by electrocardiographic recordings, including 12-lead surface electrocardiograms and 24-hour Holter recordings performed <3 months after the initial visit, and by the medical history of AF from the referring physician. New-onset AF that occurred >3 months after the initial visit was not included in the diagnosis of AF in the present study.
We confirmed HF events (HF requiring hospitalization or death with HF) that were classified into International Classification of Diseases, 10th Revision, code numbers of I50 with the medical records of our hospital or by the information obtained from follow up. We excluded events that occurred at the initial visit.
For the patients’ backgrounds, categorical and consecutive data are presented as number (percentage) and as mean ± SD, respectively. The incidence of HF events was estimated as a cumulative incidence rate using the Kaplan-Meier method ( Figure 1 ). Then, for the univariate analysis of the risk for the HF events, the relative risks of various cofactors were calculated, and the 95% confidence intervals were estimated with Poisson distributions. All cofactors showing significant univariate relations with HF events were included in a Cox regression multivariate model with coexisting organic heart diseases (including [1] valvular heart disease with moderate or greater severity, left ventricular hypertrophy [intraventricular septal or posterior wall thickness ≥14 mm], or left ventricular dysfunction [ejection fraction <50%] on echocardiography; [2] previous diagnosis of coronary artery disease by coronary angiography; [3] previous diagnosis of congenital heart disease; and [4] left ventricular noncompaction on echocardiogram), female gender, age (≥70 years), diabetes mellitus, hypertension, hyperuricemia, chronic kidney disease (estimated glomerular filtration rate <60 mg/min/m 2 ), anemia (hemoglobin level <11 g/dl), and the use of diuretics. To develop a risk-scoring system, the regression coefficients of the final logistic regression model were used to estimate the contribution of each variable to the risk estimation for HF events. This resulted in the attribution of 1 or 2 points for each variable to the score. Finally, we evaluated the predictive accuracy of the newly developed score using a receiver-operator characteristic curve, with which we tested the hypothesis that the predictive accuracy of the new score was significantly better than chance (indicated by a C-statistic of 0.5).
These analyses were performed using SPSS version 19.0 for Windows (SPSS, Inc., Chicago, Illinois). Statistical significance was set at a 2-sided p value <0.05.
Results
The characteristics of the study patients (with AF; n = 1,942) are listed in Table 1 . Patients with AF in the present study included 1,426 men (73%) and had a mean age of 66 years. Coexisting organic heart diseases were observed in approximately 1/3 of the patients with AF, and symptomatic HF was observed in approximately 20%.
| Variable | Value |
|---|---|
| Men | 1,426 (73%) |
| Age (years) | 66 ± 13 |
| Body mass index (kg/m 2 ) | 24 ± 3 |
| Coexistence of organic heart diseases ⁎ | 666 (34%) |
| Valvular heart disease | 410 (21%) |
| Coronary heart disease | 187 (10%) |
| Myocardial infarction | 77 (4%) |
| Cardiomyopathy | 195 (10%) |
| Others | 34 (2%) |
| New York Heart Association class | |
| 0 | 1,276 (66%) |
| I | 271 (14%) |
| II | 249 (13%) |
| III | 111 (6%) |
| IV | 35 (2%) |
| Coexisting diseases | |
| Diabetes mellitus | 341 (18%) |
| Hypertension † | 835 (43%) |
| Dyslipidemia ‡ | 459 (24%) |
| Hyperuricemia | 299 (15%) |
| Renal dysfunction § | 546 (28%) |
| Anemia ∥ | 94 (5%) |
| Previous cerebral infarction/transient ischemic attack | 123 (6%) |
| Medications | |
| β blockers | 618 (32%) |
| Renin-angiotensin system inhibitors | 595 (31%) |
| Angiotensin-converting enzyme inhibitors | 162 (8%) |
| Angiotensin II receptor blockers | 483 (25%) |
| Diuretics | 436 (22%) |
| Loop diuretics | 321 (17%) |
| Thiazide diuretics | 47 (2%) |
| Spironolactone | 230 (12%) |
| Antiarrhythmic drugs other than β blockers | |
| Class I antiarrhythmic drug | 523 (27%) |
| Class III antiarrhythmic drug | 51 (3%) |
| Class IV antiarrhythmic drug | 440 (23%) |
| Digitalis | 345 (18%) |
| Antithrombotic drugs | |
| Warfarin | 899 (46%) |
| Antiplatelet drugs | 820 (42%) |
⁎ Valvular heart disease, coronary heart disease, cardiomyopathy (including dilated or hypertrophic cardiomyopathy), and others (including congenital heart disease and left ventricular noncompaction).
† Systolic/diastolic blood pressure >140/90 mm Hg or antihypertensive medication use.
‡ Low-density and high-density lipoprotein and triglycerides >140, <40, and >150 mg/dl, respectively, or lipid-lowering medication use.
§ Estimated glomerular filtration rate <60 ml/min/m 2 .
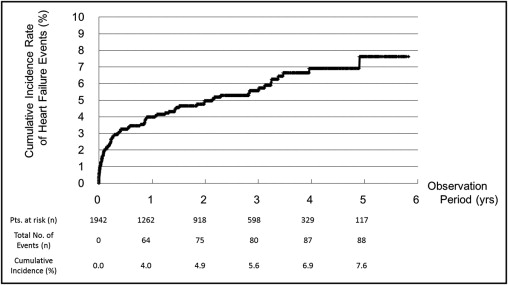
During the average follow-up period of 776 ± 623 days, hospitalization or death with HF occurred in 147 patients (7.6%); 14 patients (0.7%) died from HF. The cumulative incidence rates of HF events (hospitalization or death with HF) at 1, 3, and 5 years after the initial visit were 4.0%, 5.6%, and 7.6%, respectively ( Figure 1 ).
The relative risks for HF events of various covariates are listed in Table 2 . All covariates, except obesity and dyslipidemia, were significantly associated with the incidence of HF events. The relative risk for coexisting organic heart diseases was the strongest factor, followed by anemia and the use of diuretics.
| Variable | (Event Rate per 100 Patient-Years) | Relative Risk (95% Confidence Interval) | |
|---|---|---|---|
| With Risk Factor | Without Risk Factor | ||
| Female gender | 33 (3.0%) | 55 (1.9%) | 1.55 (1.01–2.38) |
| Age ≥70 years | 59 (3.3%) | 29 (1.3%) | 2.47 (1.59–3.85) |
| Body mass index ≥25 kg/m 2 | 20 (1.7%) | 68 (2.4%) | 0.71 (0.43–1.17) |
| Coexistence of organic heart diseases | 76 (5.8%) | 12 (0.4%) | 12.9 (7.07–23.7) |
| Diabetes mellitus | 37 (5.8%) | 51 (1.5%) | 3.80 (2.51–5.75) |
| Hypertension | 45 (2.9%) | 43 (1.8%) | 1.61 (1.06–2.43) |
| Dyslipidemia | 26 (3.0%) | 62 (2.0%) | 1.49 (0.95–2.35) |
| Hyperuricemia | 29 (5.6%) | 59 (1.7%) | 3.33 (2.15–5.14) |
| Renal dysfunction (estimated glomerular filtration rate <60 ml/min/m 2 ) | 55 (5.3%) | 33 (1.1%) | 4.70 (3.07–7.20) |
| Anemia (hemoglobin <11 g/dl) | 19 (16.6%) | 69 (1.8%) | 9.33 (5.82–14.9) |
| Use of diuretics | 59 (7.3%) | 29 (0.9%) | 8.01 (5.17–12.4) |
The multivariate Cox regression model for the incidence of HF events is listed in Table 3 . With the stepwise method using all the covariates that showed significant univariate relations with HF events, the coexistence of organic heart diseases, anemia, renal dysfunction, diabetes, and the use of diuretics were identified as the independent predictors for HF events.
| Variable | Hazard Ratio (95% Confidence Interval) | p Value |
|---|---|---|
| Heart diseases ⁎ | 5.68 (2.87–11.23) | <0.001 |
| Anemia (hemoglobin <11 g/dl) | 3.01 (1.78–5.10) | <0.001 |
| Renal dysfunction (estimated glomerular filtration rate <60 ml/min/m 2 ) | 2.34 (1.49–3.67) | <0.001 |
| Diabetes mellitus | 1.83 (1.18–2.82) | 0.006 |
| Diuretics | 1.95 (1.17–3.26) | 0.011 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


