A “learning health care system”, as outlined in a recent Institute of Medicine report, harnesses real-time clinical data to continuously measure and improve clinical care. However, most current efforts to understand and improve the quality of care rely on retrospective chart abstractions complied long after the provision of clinical care. To align more closely with the goals of a learning health care system, we present the novel design and initial results of the Veterans Affairs (VA) Clinical Assessment, Reporting, and Tracking (CART) program—a national clinical quality program for VA cardiac catheterization laboratories that harnesses real-time clinical data to support clinical care and quality-monitoring efforts. Integrated within the VA electronic health record, the CART program uses a specialized software platform to collect real-time patient and procedural data for all VA patients undergoing coronary procedures in VA catheterization laboratories. The program began in 2005 and currently contains data on 434,967 catheterization laboratory procedures, including 272,097 coronary angiograms and 86,481 percutaneous coronary interventions, performed by 801 clinicians on 246,967 patients. We present the initial data from the CART program and describe 3 quality-monitoring programs that use its unique characteristics—procedural and complications feedback to individual labs, coronary device surveillance, and major adverse event peer review. The VA CART program is a novel approach to electronic health record design that supports clinical care, quality, and safety in VA catheterization laboratories. Its approach holds promise in achieving the goals of a learning health care system.
In 2004, the Department of Veterans Affairs (VA) embarked on a national initiative to improve the quality of its cardiac care. As part of this initiative, the need to measure the quality and outcomes of patients undergoing procedures in all the VA cardiac catheterization laboratories was identified. In response, VA operational, clinical, and research leaders proposed a national clinical quality program for VA catheterization laboratories that could generate real-time clinical data to support quality-monitoring efforts and achieve the goals of a learning health care system. The new program—the VA Clinical Assessment, Reporting, and Tracking (CART) program—captures clinical information at the point of care and allows for its immediate use to support quality-monitoring programs. By using clinical data to generate both the patient record of care and the data to monitor and improve the quality of that care, separate chart abstraction efforts and maintenance of independent databases are no longer required. As a result, The CART program provides the building blocks for a “learning health care system” that can provide real-time data to drive continuous improvement of cardiac care in VA catheterization laboratories. In this manuscript, we describe CART’s design, implementation, data, and initial quality-monitoring programs.
Methods
A team of clinicians, health services researchers, and information technology developers designed the CART program. The foundation of the program is a clinical software application integrated into the VA electronic health record (EHR). When any coronary procedure (i.e., diagnostic angiogram or percutaneous coronary intervention [PCI]) in any VA catheterization laboratory is performed, the clinicians use the application to record patient and procedural data. These data are automatically recorded in the EHR as the procedural note. In addition, it is available for analysis to support quality-monitoring and research efforts, both locally and nationally.
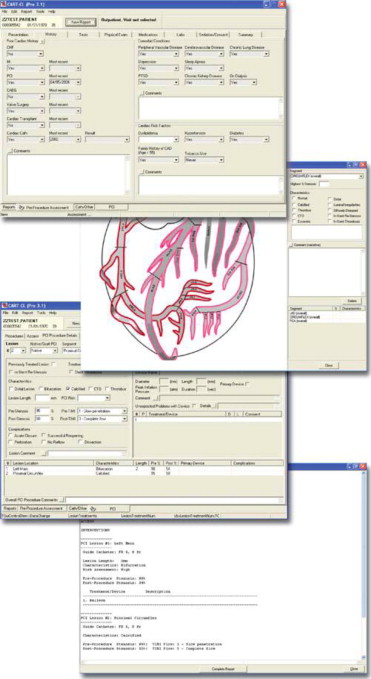
In order for the CART data to serve these multiple purposes simultaneously, standardized and comprehensive data about both patients and procedures are needed. The CART software application enables standardized clinical data entry at the point of care using data elements and definitions from the American College of Cardiology’s National Cardiovascular Data Registry, the largest cardiac clinical registry in the United States. This harmonization of data elements permits direct comparisons between VA sites as well as benchmarking and comparison of VA care with the >1,500 non-VA medical centers that participate in the National Cardiovascular Data Registry CathPCI Registry. Data elements and definitions are selected and regularly updated by a clinical advisory committee of VA interventional cardiologists to maintain clinical relevance (e.g., adoption of new techniques and procedures) and ease of use. Regular communication between the CART program leadership, National Cardiovascular Data Registry, and “front line” clinicians in the VA catheterization laboratories ensures that the system remains current. At the time of a catheterization laboratory procedure, clinicians use standardized data fields to enter discrete data elements for both preprocedural and procedural clinical notes ( Figure 1 ). To maximize efficiency, information that is already available from the EHR, such as demographics, clinical conditions, medications, vital signs, and laboratory results, are automatically imported into the preprocedural note. The clinician can also supplement this initial data with additional information as needed. After completion of the catheterization laboratory procedure, the clinician then records procedural information and outcomes, again using the standardized data fields. Once completed, standardized and comprehensive preprocedures, cardiac catheterization, and/or PCI reports are immediately available in the EHR, thus providing “real-time” information to the patient and his or her care team.
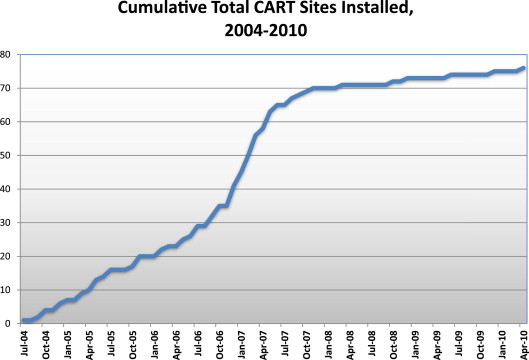
Implementation of the CART program began in 2004 and, by the end of 2010, was used to record patient and procedural data on all coronary angiographies and PCIs performed in all VA catheterization laboratories nationwide ( Figure 2 ). As of April 2014, the CART program had collected data on 434,967 catheterization laboratory procedures, including 272,097 coronary angiograms and 86,481 PCIs, performed by 801 clinicians on 246,967 patients.
High quality data are essential to the CART program’s mission of improving clinical care, quality, and research. By virtue of its design, CART establishes the groundwork for high data quality in 3 important domains—data representativeness, completeness, and validity. Data representativeness occurs when the data set is sufficiently representative of the population being studied. Because the CART program is embedded within the medical record for all VA patients receiving coronary procedures nationwide, rather than a separate data registry, it completely represents of the population it captures, namely all veterans undergoing coronary procedures at any of the VA cardiac catheterization laboratories. Data completeness in CART is facilitated by its use of nationally established data standards for recording catheterization laboratory procedural data and a user interface that facilitates easy data entry. Data validity between CART and the EHR is optimized by the tight integration of CART into clinical workflow. CART’s impact on data validity, completeness, and timeliness has resulted in significant improvements in catheterization laboratory data quality, as demonstrated in a previously published analysis.
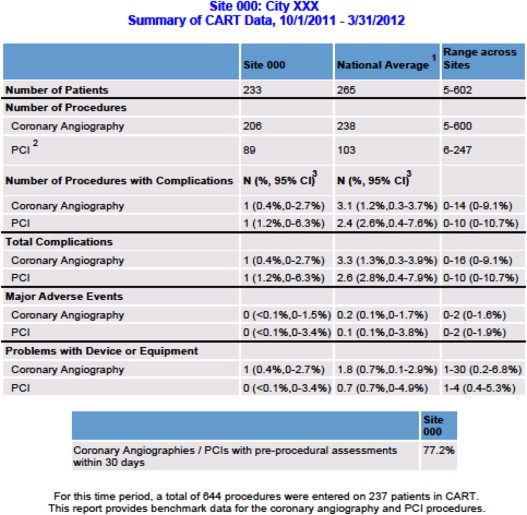
Initial quality-monitoring efforts using CART data focus on 3 areas: periodic feedback of procedural data to individual catheterization laboratories for workload tracking and evaluation, coronary device surveillance in conjunction with the U.S. Food and Drug Administration (FDA), and catheterization laboratory major adverse events (MAE) peer review. Using patient and procedural data, CART generates quality benchmark reports that are fed back to catheterization laboratory directors and leadership monthly for audit purposes. The reports detail local and national catheterization laboratory workload, completeness of data entry, and complication rates ( Figure 3 ). This reporting mechanism can assist catheterization laboratory leadership and staff in determining resource needs for workload demand, identifying deficiencies in data quality and areas for improvement and reviewing in-lab complications.
To monitor coronary devices, the CART application includes specific data fields to capture any unexpected device problems (UDP) that occur during a catheterization laboratory procedure. If a clinician notes a device problem by entering information into this data field, the CART program automatically generates an “unexpected problem with device” alert. These alerts are reviewed and triaged by the CART coordinating center. After review, the UDP alert is assigned 1 of 3 designations: level 1—problem unlikely to be related to the device; level 2—problem possibly related to the device; and level 3—problem likely related to the device. For all level 2 and 3 designations, the surveillance team conducts an investigation of the incident in conjunction with the individual catheterization laboratory provider. If the investigation uncovers a potential quality or safety issue with a device, then the CART coordination center submits a formal report to the FDA’s MedWatch reporting program. In addition, the VA and the FDA have established a formal partnership to periodically review data regarding problems or issues with cardiac devices that might lead to the early identification of potential patient safety issues. As this program continues to develop, it can serve as a model for effective surveillance medical device safety.
To conduct effective peer review of adverse events, the CART MAE program was launched in January 2011. The program continually monitors VA catheterization laboratory procedures for any in-lab deaths, strokes, or need for emergency coronary artery bypass grafting surgeries that may occur. If an event occurs, a national committee of VA interventional cardiologists conducts a formal, protected peer review of the MAE. Reviewers are specifically directed to focus on system-level issues that may have led to the MAE and provide action items to correct any identified issues. In addition, lessons learned from individual peer review are periodically shared with the broader VA catheterization laboratory community, so that sites can learn from their colleagues and potentially alter their processes of care to avoid similar MAEs.
Results
Since 2009, patient and procedural data from the CART program have been analyzed and reported annually. In fiscal year 2013 (October 2012 through September 2013), the 78 VA catheterization laboratories performed a total of 38,509 coronary angiograms in 36,789 patients, and 11,365 PCIs in 10,267 patients ( Table 1 ). The median age of patients who underwent coronary angiography was 65.2 years and 97.2% were men ( Table 2 ). Previous cardiac disease, cardiovascular risk factors, and coexisting chronic medical conditions were highly prevalent.
| Coronary Angiography | |
| Coronary Angiograms | 38,509 |
| Patients | 36,789 |
| Total number of cardiac cath labs | 78 |
| Number of coronary angiograms performed by cath lab | 458 (297-698) |
| Percutaneous Coronary Intervention (PCI) | |
| PCI Procedures | 11,365 |
| Patients | 10,267 |
| Total number of cardiac cath labs | 66 |
| Number of PCIs performed by lab | 158 (102-226) |
| Variable | Total Patients (n = 34,262) ∗ | Missing Data |
|---|---|---|
| Age (years) | 65.2 (60.6-70.1) | 0 |
| Men | 33,301 (97.2%) | 0 |
| Prior myocardial infarction | 9,791 (32.1%) | 3,759 |
| Prior percutaneous coronary intervention | 9,941 (32.7%) | 3,879 |
| Prior coronary artery bypass grafting | 5,990 (19.9%) | 4,227 |
| Prior valvular repair/replacement | 584 (2.0%) | 5,473 |
| Congestive heart failure | 8,217 (27.3%) | 4,114 |
| Hypertension | 29,136 (91.1%) | 2,289 |
| Dyslipidemia † | 28,672 (89.9%) | 2,384 |
| Current/former tobacco use | 22,124 (82.5%) | 7,428 |
| Diabetes | 15,262 (52.0%) | 4,890 |
| Obesity ‡ | 15,783 (49.3%) | 2,253 |
| Family history of coronary artery disease § | 5,934 (26.1%) | 11,558 |
| Cerebrovascular disease | 4,966 (18.8%) | 7,830 |
| Peripheral arterial disease | 5,447 (20.4%) | 7,506 |
| Chronic obstructive pulmonary disease | 7,064 (29.8%) | 9,641 |
| Depression | 5,378 (24.7%) | 11,572 |
| Chronic kidney disease | 5,139 (21.1%) | 8,989 |
| Post-traumatic stress disorder | 5,118 (24.1%) | 12,107 |
| Obstructive sleep apnea | 4,094 (20.6%) | 13,473 |
∗ Numbers/Percentages based on procedures with a matching assessment; 8,802 (15.8%) procedures have missing pre-procedural assessments.
† Defined by the National Cholesterol Education Program criteria include documentation of the following: total cholesterol greater than 200 mg/dL (5.18 mmol/l) or low-density lipoprotein (LDL) greater than or equal to 130 mg/dL (3.37 mmol/l) or high-density lipoprotein (HDL) less than 40 mg/dL (1.04 mmol/l).
‡ Defined by body mass index ≥ 30kg/m 2 .
§ Defined by coronary artery disease in any direct blood relatives (parents, siblings, children) diagnosed at age less than 55 years for male relatives or less than 65 years for female relatives.
Primary indications for coronary angiograms during this period included chest pain in 17,159 (51%), acute coronary syndrome in 6,499 (19.3%), and noninvasive evidence of ischemia in 11,111 (33.1%; Table 3 ). Obstructive coronary artery disease (i.e., >70% stenosis in ≥1 epicardial coronary vessels) was noted in a majority of patients. A total of 487 (1.2%) complications occurred during coronary angiograms, and the vast majority of these were minor and without long-term clinical sequelae (e.g., small hematomas at the vascular access site). Of the 11,365 patients who underwent PCI, 2,783 (32.2%) received the procedure to treat stable angina and 5,085 (55.1%) to treat acute coronary syndrome ( Table 4 ). Among patients with ST-segment elevation myocardial infarction with available data (n = 632), 252 (84.8%) of cases occurred within the 90-minute timeframe recommended by the American College of Cardiology/American Heart Association guidelines. A total of 657 (5.8%) complications were noted during PCI, with the vast majority of these representing minor events, such as small vascular site hematomas or transient self-limited arrhythmias during the procedure. Table 5 demonstrates selected trends in workload, patient, and procedural characteristics from fiscal year 2009 (the first year of full catheterization laboratory participation in CART) through fiscal year 2013.
| Variable | Total Angiograms (n=38,509) | Missing Data |
|---|---|---|
| Procedural Indication (not mutually exclusive) | 4,881 | |
| Chest pain | 17,159 (51%) | |
| Acute coronary syndrome | 6,499 (19.3%) | |
| Non-invasive evidence of ischemia | 11,111 (33.1%) | |
| Cardiomyopathy | 2,446 (7.3%) | |
| Valvular heart disease | 2,625 (7.8%) | |
| Other indications ∗ | 6,217 (18.1%) | |
| Primary Coronary Access | 174 | |
| Femoral | 29,181 (75.8%) | |
| Radial | 8,914 (23.2%) | |
| Other | 414 (1.0%) | |
| Coronary Artery Disease Distribution | 3,073 | |
| Left main | 100 (0.3%) | |
| Left main + 3 vessel | 1,052 (2.9%) | |
| Left main + 2 vessel | 319 (0.9%) | |
| Left main + 1 vessel | 140 (0.4%) | |
| 3 vessel | 4,393 (12.2%) | |
| 2 vessel | 4,723 (13.2%) | |
| 1 vessel | 6,379 (17.8%) | |
| Non-obstructive coronary artery disease | 7,393 (20.6%) | |
| No coronary artery disease | 4,352 (12.1%) | |
| Prior coronary artery bypass grafting | 6,807 (19.0%) | |
| Other | 199 (0.6%) | |
| Other angiogram characteristics | ||
| Left ventriculography | 12,131 (29.5%) | |
| Right heart catheterization | 5,287 (14.7%) | |
| Fractional flow reserve | 2,099 (7.4%) | |
| Intravascular ultrasound | 530 (1.4%) | |
| Intra-aortic balloon pump | 191 (0.7%) | |
| Temporary pacemaker | 63 (0.6%) | |
| Mechanical vascular closure device | 21,609 (54.1%) | |
| Contrast volume (cc) | 90 (63-130) | |
| Fluoroscopy time (min) | 6.3 (3.6-11.7) | |
| Complications and Unexpected Problems with Device | 2,176 | |
| Overall † | 487 (1.2%) | |
| In-Lab Death | 5 (0.01%) | |
| In-Lab cerebrovascular accident | 13 (0.03%) | |
| Need for emergent coronary artery bypass grafting | 3 (<0.01%) | |
| Unexpected problems with device | 226 (0.6%) | |
∗ Examples of other indications include pre-operative evaluation, post-cardiac transplant, or participation in a research study.
† Overall complications were largely minor and without long-term clinical sequelae (e.g. small hematomas at the vascular access site, transient intra-procedure arrhythmias, etc.).
| Variable | Total PCIs (n=11,365) | Missing Data |
|---|---|---|
| Procedural Indications | 2,720 | |
| Chest pain or stable angina | 2,783 (32.2%) | |
| ST-elevation myocardial infarction | 632 (7.3%) | |
| Non ST-elevation myocardial infarction | 1,999 (23.1%) | |
| Unstable Angina Pectoris | 2,392 (27.7%) | |
| Non-invasive evidence of ischemia | 146 (1.6%) | |
| Coronary stent re-stenosis | 246 (2.9%) | |
| Cardiogenic shock | 62 (0.7%) | |
| Other indications ∗ | 799 (9.2%) | |
| ST-elevation myocardial infarction door to balloon time (min) | 67 (50-84) | 335 |
| ST-elevation myocardial infarction cases with door to balloon times <90 minutes | 252 (84.8%) | |
| Number of stenoses treated | 1 (1-2) | |
| Percutaneous coronary intervention location | ||
| Left main (protected) | 243 (1.5%) | |
| Left main (unprotected) | 202 (1.4%) | |
| Left anterior descending | 5,300 (34.1%) | |
| Left circumflex | 3,696 (23.8%) | |
| Right coronary | 4,626 (29.8%) | |
| Ramus | 292 (1.9%) | |
| Other native coronary location | 78 (0.2%) | |
| Bypass Grafts | 1,114 | |
| Left internal mammary artery graft | 35 (3.2%) | |
| Saphenous vein graft | 1,060 (95.2%) | |
| Right internal mammary artery graft | 4 (0.3%) | |
| Radial artery graft | 14 (1.3%) | |
| Other bypass graft | 1 (<.01%) | |
| Percutaneous coronary intervention risk | ||
| High | 5,674 (44.8%) | 3,371 |
| Non-high | 6,985 (55.2%) | |
| Percutaneous coronary intervention timing † | ||
| Ad hoc | 9,832 (95.6%) | 1,079 |
| Staged | 454 (4.4%) | |
| Percutaneous coronary intervention device | ||
| Drug-eluting stent | 12,765 (81.2%) | |
| Bare-metal stent | 1,670 (10.6%) | |
| Balloon angioplasty | 1,283 (8.2%) | |
| Percutaneous coronary intervention success | ||
| Overall success ‡ | 15,379 (95.9%) | 651 |
| Pre-percutaneous coronary intervention stenosis | 90 (80-95) | 1,073 |
| Post-percutaneous coronary intervention stenosis | 0 | 1,151 |
| Contrast and radiation exposure | ||
| Contrast volume (cc) | 207 (150-300) | |
| Fluoroscopy time (min) | 18.9 (11.9-30) | |
| Complications | ||
| Overall § | 657 (5.8%) | |
| In-lab death | 8 (0.07%) | |
| In-lab cerebrovascular accident | 1 (<0.01%) | |
| Need for emergent coronary artery bypass grafting | 4 (0.03%) | |
| Unexpected problems with device | 77 (0.7%) | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


