The purpose of this meta-analysis was to compare 1 year mortality and major adverse cardiovascular and cerebrovascular events between transfemoral (TF) transcatheter aortic valve implantation (TAVI) and transapical (TA) TAVI performed using Edwards valves. PubMed, Embase, and the Cochrane Center Register of Controlled Trials were searched for studies published from January 2000 through March 2014. Seventeen studies met the inclusion criteria and were included in the analysis. This meta-analysis included total of 2,978 patients with severe aortic stenosis not eligible for traditional surgical procedures who underwent TF TAVI (n = 1,465) or TA TAVI (n = 1,513). End points were in-hospital, 30-day, and 1-year all-cause mortality, stroke, myocardial infarction, major bleeding, and major vascular complications. Odds ratios (ORs) with 95% confidence interval (CIs) were computed, and p values <0.05 were considered to indicate statistical significance. The studies were homogenous for all outcomes except 1-year mortality. There was no significant difference between the TF and TA TAVI groups for 1-year mortality (OR 0.64, 95% CI 0.34 to 1.2, p = 0.16), incidence of stroke (OR 1.14, 95% CI 0.76 to 1.71, p = 0.52), incidence of myocardial infarction (OR 0.62, 95% CI 0.23 to 1.7, p = 0.35), and incidence of bleeding events (OR 0.76, 95% CI 0.51 to 1.14, p = 0.19). Thirty-day all-cause mortality was significantly less with TF TAVI compared with TA TAVI (OR 0.59, 95% CI 0.45 to 0.76, p <0.0001). Major vascular events were significantly higher in the TF TAVI group compared with the TA TAVI group (OR 4.33, 95% CI 3.14 to 5.97, p <0.00001). In conclusion, the results of this meta-analysis of 2,978 patients revealed that TA TAVI had similar 1-year major adverse cardiovascular and cerebrovascular events, fewer major vascular complications, but higher 30-day mortality compared with TF TAVI. In patients with contraindications to TF TAVI, TA TAVI is a reasonable option, although further randomized trials are warranted for evaluating long-term clinical outcomes between TF and TA TAVI.
Severe aortic stenosis (AS) is the most common form of heart valve disease that affects the elderly population when not associated with genetic abnormalities. Symptomatic AS or a left ventricular ejection fraction <50% is an indication for valve replacement. Because nearly 1/3 of patients with severe AS are denied traditional surgical aortic valve replacement (SAVR) because of co-morbidities and expected poor surgical outcomes, transcatheter aortic valve implantation (TAVI) has been compared with surgical replacement in the past. Benefits of TAVI in high-risk patients who are not eligible for surgical replacement are well documented. A recent meta-analysis compared TAVI with SAVR and demonstrated that TAVI is superior to SAVR for major bleeding complications and noninferior to SAVR for postprocedural myocardial infarctions (MIs) and cerebrovascular events. TAVI is performed using transfemoral (TF), transapical (TA), subclavian, and transaortic approaches. TF TAVI is a retrograde approach, whereas TA TAVI is an anterograde approach with apical minithoracotomy, which poses more risk for mortality and morbidity. TF TAVI is the preferred approach, given less inherent risk for periprocedural complications by avoiding more invasive steps such as minithoracotomy and left ventricular puncture. The purpose of this meta-analysis was to compare mortality and major adverse cardiovascular and cerebrovascular events (MACCEs) between TF and TA TAVI performed using Edwards valves.
Methods
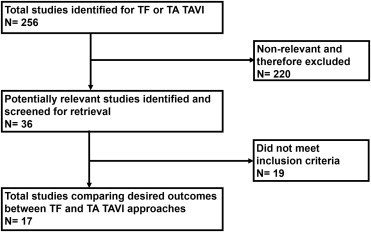
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses and Meta-Analysis of Observational Studies in Epidemiology statements for reporting systematic reviews. General guidelines from the Cochrane Handbook for Systematic Reviews of Interventions , version 5.0.2 were used in developing methods, and the meta-analysis was conducted in adherence to these guidelines. We searched the National Library of Medicine PubMed, National Institutes of Health clinical trials registry ( ClinicalTrials.gov ), and the Cochrane Central Register of Controlled Trials to include clinical studies comparing mortality and MACCEs between TF and TA TAVI for severe AS. Studies were included if conducted from January 2000 through March 2014. The key words used for searching studies were “transcatheter,” “aortic valve,” “aortic stenosis,” “aortic valve replacement,” “TAVI,” “transfemoral,” “transapical,” and “transcatheter aortic valve replacement (TAVR).” In addition to our computerized search, we manually reviewed the reference lists and related links of all retrieved reports to complete our search. Two independent investigators (HBP and VL) reviewed all titles from the search results, and reports were selected for final data extraction. The selection process is outlined in Figure 1 .
Studies comparing outcomes between TF and TA TAVI procedures using Edwards valves were included in this meta-analysis. To be selected for analysis, a study had to meet all inclusion criteria: (1) the study must compare TF and TA TAVI performed in patients with severe AS, and (2) the study must report ≥1 of the following MACCE outcomes: all-cause mortality (in-hospital, 30-day, and 1-year), MI, stroke, major vascular events, and major bleeding events. Studies that did not meet any of these criteria were excluded.
After identifying all relevant reports, we extracted data from each study, including investigators, year, design, sample size, follow-up duration, types of valves placed, types of TAVI procedures, baseline clinical characteristics of the patient population, baseline logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) representing perioperative risk, and postprocedural outcomes. End points extracted were all-cause mortality (in-hospital, 30-day, and 1-year), MI, stroke, major vascular events, and major bleeding events. The main objective of this study was to evaluate mortality and MACCE outcomes. Two reviewers (HBP and VL) independently extracted data and assessed outcomes. Interrater agreement was 90%, and disagreements were resolved by consensus.
The quality of the included studies in present analysis was assessed using the Newcastle-Ottawa Scale for quality assessment of cohort studies ( http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm ). Briefly, studies were quoted using prespecified items on patient selection (representativeness and selection of patients, ascertainment of exposure), comparability of cohorts, and assessment of outcomes (recording, adequacy of follow-up). Ratings for each item were added to provide a study quality score (maximal score 9). Two independent reviewers performed Newcastle-Ottawa Scale grading. Discrepancies were solved by consensus.
The mean difference or odds ratio (OR) across all studies with the corresponding 95% confidence interval (CI) was calculated for each end point by using RevMan version 5.1 (The Cochrane Collaboration, Copenhagen, Denmark) for continuous and dichotomous outcomes, respectively. Heterogeneity of the studies was assessed for each end point. Studies that were homogenous for an end point were analyzed by using the Mantel-Haenszel fixed-effect model, and studies that were heterogenous for an end point were analyzed by using the random-effect model. Publication bias was also analyzed using a funnel-plot method. In addition, meta–regression analyses were conducted to determine whether comparisons of mortality and MACCEs between TF and TA TAVI were modulated by prespecified factors, including gender, chronic obstructive pulmonary disease, coronary artery disease (CAD), Society of Thoracic Surgeons (STS) score, and logistic EuroSCORE. Comparison of MI outcomes between TF and TA TAVI was not performed by prespecified factors, because of inadequate data. These meta–regression analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina). A p value <0.05 was considered to indicate statistical significance.
Results
Seventeen studies met the inclusion criteria and were included for the analysis. The search and selection process is shown in Figure 1 . Although we searched for studies published through March 2014, the most recent study that met our inclusion and exclusion criteria was published in 2012. Study overview and baseline patient characteristics are listed in Table 1 . This meta-analysis included total of 2,978 patients with severe AS who were not eligible for SAVR and underwent TF TAVI (n = 1,465) or TA TAVI (n = 1,513). Follow-up was nearly 99% complete for included subjects in all studies. Study quality assessment was provided in Table 1 . Logistic EuroSCORE was compared between the 2 groups in all but 4 studies. Publication bias and heterogeneity for each outcome are listed in Table 2 .
| Study | Design | Patient characteristics | Total population | Mean follow-up days | Newcastle-Ottawa Scale | End points | ||
|---|---|---|---|---|---|---|---|---|
| Transfemoral Mean (SD) or (%) | Transapical Mean (SD) or (%) | TF | TA | |||||
| Osten 2009 N=46 | Retrospective analysis of prospective database | Age 82(9), F 38%, LES 24.2, STS 7.2,CAD 81%, DM 25%,CKD 75%,PAD 44%,COPD 13%, | Age79(7), F66%, LES25.9, STS 9.5, CAD 67%, DM 33%,,CKD 57%,PAD 43%,COPD 33%, | 16 | 30 | >365 ∗ | 9 | IHM, 30D mortality, MI, bleeding events |
| Rodes-Cabau 2010 N=339 | Retrospective | Age 83(8), F 43.4%, STS 9(5.8), LVEF 55(14) ,CAD 67.9%,DM 22.8%,PAD 19.1%,COPD 27.8%, | Age 80(8), F 65.5%, STS 10.5(6.9), LVEF 56(14) ,CAD 70.1%,DM 23.7%,PAD 50.3%,COPD 31.1%, | 168 | 177 | 30 | 8 | 30D mortality, stroke, MI, bleeding events |
| Thomas 2010 N= 1038 | Retrospective | Age 81.7(6.7), F 55.2%, LES 25.7(14.5) ,CAD 47.4%,CKD 25.5 %,PAD 10.9%,COPD 25.4%, | Age 80.7(7), F 56%, LES 29.1(16.3) ,CAD 56%,CKD 32.5 %,PAD 27.5%,COPD 29.4%, | 463 | 575 | 30 | 8 | 30D mortality, stroke, MVC |
| Bosmans 2011 N=328 | Retrospective analysis of prospective registry | Age 84(5), LES 29(15),LVEF 51(16) ,PVD 4%,DM 10%, | Age 82(6), LES 33(17), LVEF 51(14) , PVD 34%,DM 19% | 99 | 88 | 365 | 8 | 30D mortality, 1yr mortality, stroke |
| Ewe 2011 N=104 | Retrospective | Age 82(7), F 53.3 %, LES 20.1(11.7), STS 8.5(3.8) ,DM 28.9%,CKD 22.2 %,PAD 11.1%,COPD 24.4%, | Age 79.4(8.3), F 47.5%, LES 22.6(11.9), STS 8.3(3.5), ,DM 27.1%,CKD 22 %,PAD 67.8%,COPD 28.8%, | 45 | 59 | 485 | 9 | IHM, 30D mortality, 1yr mortality, stroke, MVC, bleeding events |
| Eltchaninoff 2011 N=244 | Retrospective analysis of prospective registry | Age 83.2(7.3), F 44.1%, LES 25.6(11.3), STS 17.4(11.3), LVEF 47(14) ,CAD 35.8%,DM 25.3%,PAD 4.2%, | Age 82.1(7.3), F 35.7%, LES 26.8(11.6), STS 18.4(12.1), LVEF 54(12) ,CAD 46.5%,DM 25.3%,PAD 11.3%, | 95 | 71 | 30 | 9 | 30D mortality, stroke, MVC |
| Johansson 2011 N=40 | Retrospective | Age 83(6), F 50%, LES 25.6(15),CAD %,DM 10%,CKD 10%,PAD 50%,COPD 10%, | Age 80(6), F 50%, LES 23.5(17) ,CAD %,DM 30%,CKD 3%,PAD 47%,COPD 40%, | 10 | 30 | 365 | 9 | IHM, stroke, MVC |
| Thomas 2011 N= 1038 | Retrospective | Age 81.7(6.7), F 55.2%, LES 25.7(14.5) ,CAD 47.5%,CKD 25.5%,PAD 10.6%,COPD 24.6%, | Age 80.7(7), F 56%, LES 29.1(16.3) ,CAD 55.1%,CKD 32.5%,PAD 28.0%,COPD 29.9%, | 463 | 575 | 365 | 7 | 1yr mortality |
| Al-Attar 2009 N=50 | Prospective | Age 83(6), F 17(49%), LES 26(14), STS 15(6), LVEF 50 (16) ,CAD 46%,DM 17%,CKD 26%,PAD 11.5% | Age 83(10), F 6(40%), LES 30(12), STS 19(9), LVEF 45(13) ,CAD 80%,DM 27%,CKD 53%,PAD 26.7%, | 35 | 15 | >365 ∗ | 9 | HM, stroke, MVC |
| Himbert 2009 N=104 | Prospective | Age 82(7), F 51%, LES 25(13), STS 15(7), LVEF 52(16), CAD 49%,CKD 31%,PAD 8%,COPD 27%, | Age 82(10),F 33%, LES 28(13), STS 18(9), LVEF 48(13) ,CAD 87%,CKD 52%,PAD 30%,COPD 26%, | 51 | 24 | >365 ∗ | 9 | IHM, 1yr mortality, stroke, MVC |
| Webb 2009 N= 168 | Prospective | Age 85(IQ79-88), F 48(42.5%), LES 25(16-37), STS 8.7(6-12) ,CAD 64.6%,DM 26.5%,CKD 12.4%,PAD 15.9%,COPD 22.1%, | Age 83(76-87), F 33(60%), LES 35(20-50.3), STS 10.3(6.8-17.7) ,CAD 74.5%,DM 16.4%,CKD 10.9%,PAD 76.4%,COPD 18.2%, | 113 | 55 | >365 ∗ | 8 | 30D mortality, 1yr mortality, stroke, MVC |
| Dworakowski 2010 N=151 | Prospective | Age 83(0.8), F 24(36%), LES 19.4(1.1) ,CAD 38.8%,DM 26.9%,PAD 10.4%,COPD 22.4%, | Age 82.2(0.8), F 45(54%), LES 23.4(1.5) ,CAD 47.6%,DM 20.2%,PAD 35.7%,COPD 31.0%, | 67 | 84 | 365 | 8 | 30D mortality, 1yr mortality, stroke, MVC, MI |
| Gurvitch 2011 N=270 | Prospective | Age 83(8), STS 8.5, F 26(41%), No LES,CAD 75%,PAD 16%,COPD 28%, | Age 81(7), F62(63%), STS 11.2, No LES,CAD 78%,PAD 66%,COPD 22%, | 169 | 101 | 30 | 8 | IHM, 30D mortality, stroke. MVC |
| Lefevre 2011 N=130 | Prospective | Age 82.3(5.2), F 60.7%, LES 25.7(11.5), STS 11.3(6.1), LVEF 52.9(17.8) ,CAD 54.1%,DM 34.4%,CKD 36.1%,PAD 16.4%,COPD 49.2%, | Age 81.9(5.7), F 50.7%, LES 33.8(14.4), STS 11.8(6.8), LVEF 52.8(14.6) ,CAD 65.2%,DM 29.0%,CKD 46.4%,PAD 49.3%,COPD 34.8%, | 55 | 65 | 365 | 8 | 30D mortality, 1yr mortality, stroke,, MVC, ,MI, bleeding events |
| Nielson 2011 N=100 | Prospective | Age 83.2(7.6), F 14(54.2%), LES 15.9(9.4),DM 4%,PAD 8%,COPD 29%, | Age 80.6(6.7), F 43(56.6%), LES 21.5(13.5),DM 16%,PAD 12%,COPD 28%, | 24 | 76 | >365 ∗ | 8 | 30D mortality, 1yr mortality, stroke, MI |
| Wenaweser 2011 N=70 | Prospective | Age 83.9(4), F 16(59%), LES 22.8(13), STS 6.3(4) ,CAD 51.9%,DM 29.6%,PAD 11.1%, | Age 78.1(9.6), F 19(44%), LES 26.1(14.3), STS 6.3(5.5) ,CAD 79.1%,DM 34.9%,PAD 39.5%, | 27 | 43 | 365 | 8 | 30D mortality, stroke, MI, bleeding events |
| Zhao 2012 N=48 | Prospective | Age 83.4(7.9), F 15(53.6%), LES 20.6(15.8), LVEF 54.1(18.6) ,CAD 60.7%,DM 20.8%,CKD 3.6%,PAD 25.0%,COPD 28.6%, | Age 82.3(5), F 7(36%), LES 17.3(16.1), LVEF 61.9(5.1) ,CAD 75%,DM 25.0%,CKD 5.0%,PAD 40.0%,COPD 10%, | 28 | 20 | 30 | 9 | 30D mortality |
∗ Latest follow-up mentioned in the study was 1 year. Patients were subsequently followed-up on a yearly basis; however the final follow-up duration was not mentioned.
| Outcomes | Chi- Square | df | p-Value | I Square (%) | Results | Publication Bias |
|---|---|---|---|---|---|---|
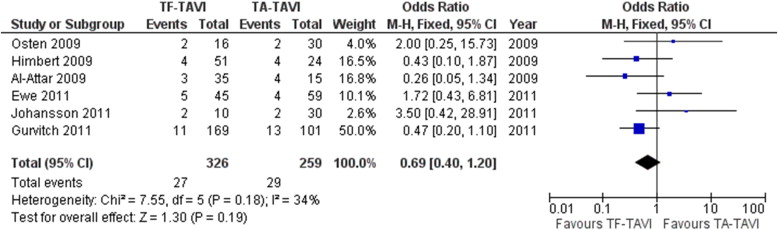
| In-hospital mortality | 7.55 | 5 | 0.18 | 34 | Homogeneous | None |
| 30 day mortality | 9.67 | 12 | 0.64 | 0 | Homogeneous | None |
| 1 year mortality | 39.08 | 7 | <0.00001 | 82 | Heterogeneous | Yes |
| Stroke | 10.47 | 13 | 0.66 | 0 | Homogeneous | None |
| Myocardial infarction | 0.61 | 3 | 0.9 | 0 | Homogeneous | None |
| Major vascular complications | 15.18 | 9 | 0.09 | 41 | Homogeneous | None |
| Major bleeding | 2.89 | 4 | 0.58 | 0 | Homogeneous | None |
In-hospital mortality was not significantly different between the TF and TA TAVI groups (p = 0.19; Figure 2 ). No significant differences in in-hospital mortality between TF and TA TAVI were identified when modulated by the effects of the prespecified factors gender, chronic obstructive pulmonary disease, CAD, STS score, and logistic EuroSCORE. Thirty-day all-cause mortality was significantly lower with TF TAVI compared with TA TAVI (p <0.0001; Figure 3 ). Similar significant differences were identified when modulated by gender (p = 0.004), CAD (p = 0.005), and logistic EuroSCORE (p = 0.04). There was no significant difference in all-cause mortality at 1 year between TF and TA TAVI (p = 0.16; Figure 4 ), but when modulated by logistic EuroSCORE, all-cause mortality at 1 year was significantly lower with TF TAVI compared with TA TAVI (p = 0.0011). The incidence of stroke was not significantly different between the TF and TA TAVI groups (p = 0.52; Figure 5 ), with no significant differences identified when modulated by the effects of the prespecified factors gender, CAD, STS score, and logistic EuroSCORE. Similarly, the incidence of MI was not statistically significant between the TF and TA TAVI groups (p = 0.35; Figure 6 ). Major vascular events were significantly more frequent in the TF TAVI group compared with the TA TAVI group (p <0.00001; Figure 7 ), and similar significant differences were identified when modulated by gender (p = 0.001), CAD (p = 0.0002), STS score (p = 0.03), and logistic EuroSCORE (p = 0.01). Bleeding events between the TF and TA TAVI groups were not significantly different (p = 0.19; Figure 8 ), with no significant differences identified when modulated by the effects of prespecified factors.