INDICATIONS FOR CARDIAC RESYNCHRONIZATION THERAPY
Case presented by:
A 67-year-old female is referred to a heart failure clinic for further management. She was initially diagnosed with a nonischemic cardiomyopathy 2 years prior. Coronary angiography, at that time confirmed the nonischemic etiology, showing only minimal plaque disease. She also has a history of an isolated episode of atrial fibrillation (AF). A recent echocardiogram demonstrated global hypokinesis with an ejection fraction (EF) of 15%. Left ventricular (LV) diameter at end-diastole measured 6.8 cm (normal 3.1–5.6 cm). Her cardiac medications at time of referral included carvedilol, losartan, spironolactone, and furosemide.
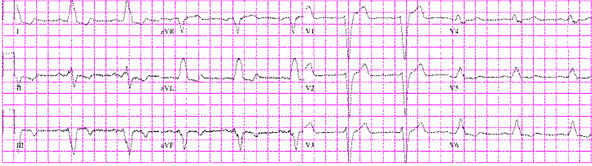
Clinically, the patient indicates that she has had a decline in her functional status over the past few months after an initial improvement with the initiation of therapy. Currently she describes significant, limiting dyspnea after 2 blocks of walking on level ground or after climbing one flight of stairs. She also has some worsening abdominal distention and lower extremity edema. Her ECG is shown in Figure 59.1.
Figure 59.1. Patient’s ECG at presentation.
A.New York Heart Association (NYHA) functional class.
B.LV end-diastolic dimension.
C.History of medical therapy for heart failure.
D.Both A and C.
Question No. 2: Which of the following ECG features predict response to CRT?
A.Absence of atrioventricular (AV) conduction delay.
B.A prolonged QRS duration.
C.Left-axis deviation.
D.Absence of Q waves.
Question No. 3: Which of the following preimplant study findings would prompt an evaluation for CRT?
A.Electrocardiographic monitoring displaying chronic right ventricular (RV) pacing.
B.Findings consistent with a nonischemic etiology on cardiac magnetic resonance imaging.
C.Areas of active ischemia on stress imaging or coronary angiography.
D.Evidence of elevated filling pressures on echocardiography or cardiac catheterization.
Question No. 4: Which of the following is a contraindication to CRT upgrade?
A.Existing dual-chamber pacing.
B.A preexisting implantable cardioverter-defibrillator (ICD).
C.Both A and B.
D.Neither A or B.
Discussion
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree