CARDIAC IMPLANTABLE ELECTRONIC DEVICES: TIMING CYCLES, SENSING, AND TROUBLESHOOTING DURING PACING
Cases presented by:
First Case
Cardiac implantable electronic devices (CIEDs) are named after their primary functions; they are pacemakers and defibrillators. The first permanent pacemakers (PPMs) in the 1960s were used only for extreme bradycardia and had only one function, to pace continuously, asynchronously, at a single rate, using a single lead placed in the ventricle. Implantable cardioverter-defibrillators (ICDs) initially had no pacing function, but later had backup emergency ventricular pacing at 30 bpm and now have fully functional single- or dual-chamber pacemakers.
Today, pacemakers and defibrillators are combined in devices with highly technical advances in the pacemakers’ specifications with multiprogrammable and automated parameter features and multiple lead-placement capabilities. The more common automatic features of these physiological CIEDs are activity responses, capture management, and sensitivity adjustment. Because of the many automated features, the follow-up of patients with CIED requires diligent review of the patients’ symptoms, rhythms, and device functioning as well as familiarity with the device-specific manufacturer’s algorithms for these automated functions, keeping in mind that physiological functioning in a PPM or ICD is tied to these specific “robotic” controls whereas the patient is not. In the end, basics of CIEDs hold true for sensing and capture, battery longevity, and patient safety, and are usually evidenced by single beat-to-beat measures. Currently used abbreviations for pacemakers are depicted in Table 54.1.
Table 54.1. Abbreviations for Pacing Modes.
| Pacing Mode | Chamber Paced | Chamber Sensed | Mode of Response |
| VOO | Ventricle (V) | None | Asynchronous (continuous ventricular pacing). |
| AOO | Atrium (A) | None | Asynchronous (continuous atrial pacing). |
| VVT | V | V | Paces the ventricle on demand for pauses < low, escape rate. Triggered (paces in the QRS [R], marking R-wave detection). |
| VVI | V | V | Paces the ventricle on demand for pauses < low, escape rate. Inhibited on R-wave detection (holds V stimulus when R wave detected). |
| AAI | A | A | Paces the atrium on demand for pauses < low, escape rate. Inhibited (holds stimulus when P wave detected). |
| VAT | V | A | Paces the ventricle on demand for pauses < low, escape rate. Triggered (paces the ventricle following P-wave detection). |
| VDD | V | A and V | Paces the ventricle on demand for pauses < low, escape rate. Triggered (paces V following P-wave detection at a programmed sensed AV [SAV] interval); does not pace the atria. Inhibited (holds V stimulus when R wave detected). |
| VDI | V | A and V | Paces the ventricle on demand for pauses < low, escape rate. Inhibited (holds V stimulus when R wave detected). Inhibited (senses atrial activity only when P wave detected). |
| DDI | A and V | A and V | Paces the ventricle on demand for pauses < low, escape rate. Paces the atrium on demand for pauses < low, escape rate – the paced AV interval = VA interval. Inhibited (holds V stimulus when R wave detected). Inhibited (holds stimulus when P wave detected); does not initiate AV delay and AV sequential pacing on P-wave sensing. |
| DDD | A and V | A and V | Paces the ventricle on demand for pauses < low, escape rate; paces the atrium on demand for pauses < low, escape rate – the paced AV interval. Triggered (paces the ventricle following P-wave detection at a programmed SAV interval). Inhibited (holds stimulus when P wave detected). Inhibited (holds stimulus when R wave detected). |
| DDD-MS (Mode switch) | A and V | A and V | See DDD above. Atrial pacing is inhibited by rapid P-wave detection (eg, atrial fibrillation) and device automatically behaves in VDI mode. |
Beats per minute (bpm) to cycle length (CL) conversion in milliseconds:
1 second = 1000 ms
60 seconds per minute × 1000 ms = 60,000 ms
60,000 ms/bpm = millisecond CL
60,000 ms/CL = bpm heart rate.
Case Description
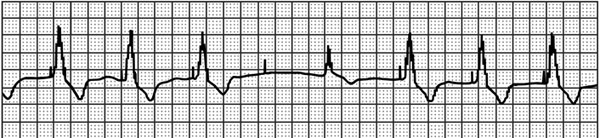
A 68-year-old male with persistent, permanent atrial fibrillation presents with complaints of intermittent palpitations and lightheadedness for the past week. His VVI (see Table 54.1 for description of pacing mode abbreviations) pacemaker (PPM) was placed 5 years ago at the left pectoral region and was programmed to 72 ppm (pulses per minute). His updated history indicates that he was placed on beta-blocker and angiotensin-converting enzyme (ACE) inhibitor for congestive heart failure 1 month ago, and had arthroscopic surgery on his left shoulder 2 months ago, soon after his last pacemaker check. Current rhythm strip is shown in Figure 54.1.
Figure 54.1. Patient’s presenting rhythm-recorded strip.
Question No. 1: Your interpretation of the strip is:
A.Failure to capture the ventricle, appropriate VVI sensing of the escape beat. Possible fractured ventricular pacing wire.
B.Failure to capture the ventricle, appropriate VVI sensing of the escape beat. Possible loose set screw with intermittent contact and intermittent capture loss.
C.Failure to capture the ventricle, appropriate VVI sensing of the escape beat. Possible threshold rise from medication/device interaction.
D.Normal PPM behavior with isoelectric QRS resulting from fusion of intrinsic and paced complexes.
E.Normal PPM behavior with Recommended Replacement Time (RRT) Indices.
Discussion
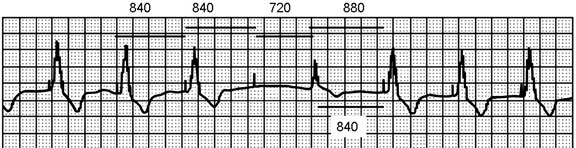
The 4 repetitive 840-ms cycles of pacing stimuli can be measured as consistent. There is no ventricular response to the fourth stimulus (failure to capture), and 720 ms later, the patient has an escape beat (1560 ms from preceding QRS), which is sensed appropriately, resulting in resetting of the escape interval and a subsequent fifth pacemaker stimulus 880 ms after the beginning of the intrinsic QRS.
Figure 54.2. Solid bars mark the intervals. Low pacing rate cycles of 840 ms are seen for three pacing cycles. The escape beat (fifth QRS) ends the open sensing period of the pacemaker at about 720 ms from the fourth pacemaker spike that did not capture the ventricle. The next pacemaker spike is 880 ms from the onset of the escape beat QRS indicating that the intrinsic QRS was sensed by the endocardial lead at least 40 ms after it was inscribed on the surface ECG recording.
Late threshold rises are either clinical or mechanical lead failures. Clinically, medication changes (beta-blocker), metabolic or electrolyte imbalances, or a substrate change with myocardial fibrosis or infarction are differentials.1 This patient’s history did not reflect acute ischemic or infective process, but there was a progression to his cardiac disease and added medication therapy. In addition to beta-blockers, several medications can increase stimulation threshold with loss of capture; most antiarrhythmics will alter thresholds but usually only at very high serum levels. Type I antiarrhythmic drugs, flecainide, procainamide, and quinidine raise thresholds. Threshold rises can be seen in metabolic conditions such as hypercarbia, hyperglycemia, hyperkalemia, hypoxemia, and acidosis or alkalosis.2 ACE inhibitor-induced hyperkalemia can occur in the setting of marginal renal function.3 Generally these effects are seen in the older adult population, but serum electrolyte, serum urea nitrogen, and creatinine would be steps toward the differential diagnosis. In contrast, corticosteroids and catacholamines can reduce stimulation thresholds. Daily fluctuations of catecholamine levels can produce higher stimulation thresholds during sleep and after eating and decreased thresholds while awake, active, and exercising. This patient presents to the clinic, awake with intermittent capture. With the pacemaker device output close to the patient’s capture threshold, more frequent episodes of failure to capture may be likely, especially at night.
Threshold rises that occur within weeks or a few months after implant are usually secondary to lead maturation, encapsulation, and polarization processes. This type of threshold rise due to the inflammatory process is reduced with the use of steroid-eluting electrodes and now rarely noted.
Points to Consider in Differential Diagnosis
A1: Possible fractured ventricular pacing wire
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree