NONINVASIVE TESTING FOR SUDDEN CARDIAC DEATH
Case presented by:
Mr. X was a 69-year-old Caucasian male with mitral valve disease and end-stage renal disease who later developed atrial fibrillation (AF) and a chronic nonischemic cardiomyopathy. His cardiac history started during 1999. He underwent mitral valve replacement for severe mitral regurgitation with a mechanical prosthesis. His left ventricular ejection fraction (LVEF) deteriorated over several years coincident with the development of persistent AF. In 2006, his LVEF measured 35% despite good ventricular rate control. He had New York Heart Association (NYHA) class II congestive heart failure (CHF). His AF was successfully treated by catheter ablation in 2006 after antiarrhythmic drugs failed. He was noninducible for sustained ventricular tachycardia (VT). Six months later, his EF was unchanged and he remained in class II CHF. A 24-hour Holter monitor revealed normal sinus rhythm with only occasional premature ventricular contractions.
Our patient was felt to be a candidate for implantable cardioverter-defibrillator (ICD) treatment based upon Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) indications, but he was undecided and reluctant. He therefore underwent microvolt T-wave alternans (MTWA) testing in 2007 for additional SCD risk stratification. The result was abnormal or positive (high risk for SCD). ICD treatment was recommended, however, other medical problems delayed implantation. He was having difficulty with his right-sided atrioventricular fistula and was being evaluated for a new left-sided fistula. He developed a large spontaneous left chest-wall hematoma that prevented implantation. He suffered SCD 6 months after his T-wave alternans test.
A.True.
B.False.
A.Patients with unexplained recurrent palpitations.
B.Post-myocardial infarction (MI) patients with left ventricular dysfunction.
C.Patient with CHF.
D.Preoperative arrhythmia evaluation of patients for noncardiac surgery.
E.Patients with idiopathic hypertrophic cardiomyopathy.
A.True.
B.False.
A.True.
B.False.
Discussion
The unresolved public health challenge of predicting and preventing SCD remains a source of frustration. Accurate identification of individuals who are likely to die of cardiac arrhythmias would allow more selective use of ICDs to prevent SCD. Significant research energy has been spent in attempts to find noninvasive tests that can separate individuals into high- and low-risk groups.
Patients with low LVEF have consistently fallen into a high-risk category. Current guidelines reflect Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II) findings, recommending ICD therapy for primary prevention of SCD in patients with ischemic heart disease and an LVEF of ≤ 30% without requiring other tests to guide therapy. As we know from MADIT-II,1 post-MI patients with LVEF of ≤ 30% are at high risk and benefit from ICD therapy. Similarly, SCD-HeFT2 showed that patients with reduced LVEF (≤ 35%) in the setting of CHF should be considered for ICD therapy to prevent SCD.
While LVEF has been the most consistent risk factor identified, there are well-recognized limitations to this approach. For example, there is often significant variation in LVEF determination based on the imaging modality utilized. In addition, the majority of SCD events occur in patients with ischemic heart disease but more preserved LVEF. Lastly, most patients who receive a primary prevention ICD based on low LVEF never require therapy from the device. For example, in SCD-HeFT,2 81% of patients in the ICD group did not use their device over 5 years of follow-up. The absolute risk reduction in the ICD group was a modest 7.2%. In MADIT-II,3 the number-needed-to-treat to avoid one death over a 20-month period was 17.9. For these reasons, there has been keen interest to identify other means of identifying patients at risk for SCD that could potentially complement LVEF.
Three such noninvasive tests, beyond LVEF, evaluated to date measure:
- Ventricular ectopy (long-term ambulatory ECG monitoring).
- Slowed or fragmented conduction (signal-averaged ECG).
- Variations in ventricular repolarization (T-wave alternans).
Ambulatory ECG Monitoring
Ambulatory ECG monitoring is the continuous recording of ECG signals from patients while they are at home and engaged in routine daily activities. These noninvasive diagnostic tests are efficacious and cost-effective in the right clinical scenario. Many patients can have asymptomatic rhythm disturbances (such as nonsustained VT), and ambulatory monitoring may help define the existing burden of the arrhythmia. Ambulatory ECG monitoring has been used to estimate prognosis in patients with ischemic4 or nonischemic cardiomyopathy.
The American College of Cardiology/American Heart Association (ACC/AHA), in collaboration with the North American Society of Pacing and Electrophysiology, have developed guidelines for the use of ambulatory ECG.5 The guidelines include recommendations for the evaluation of symptoms of cardiac arrhythmias; for risk assessment in patients who have suffered a MI, have CHF or have hypertrophic cardiomyopathy; for the evaluation of antiarrhythmic therapy, pacemaker or ICD function; and for the evaluation of possible myocardial ischemia.
Three groups may benefit from ambulatory ECG to identify increased risk of future cardiac events (class IIb indication): patients with idiopathic hypertrophic cardiomyopathy, patients with CHF, and post-MI survivors with reduced ejection fraction.
Signal-averaged Electrocardiography
Signal-averaged electrocardiography (SAECG) is a technique that averages multiple QRS complexes that are then digitalized, filtered, and further processed with spectral analysis to eliminate the background noise and detect ventricular late potentials (LPs). These LPs are low-amplitude, high-frequency signals representing areas of delayed myocardial activation due to slowed conduction from either regions of scar or fibrosis. Therefore, the LP signal represents the electrical substrate for reentry and VT (Figure 4.1).
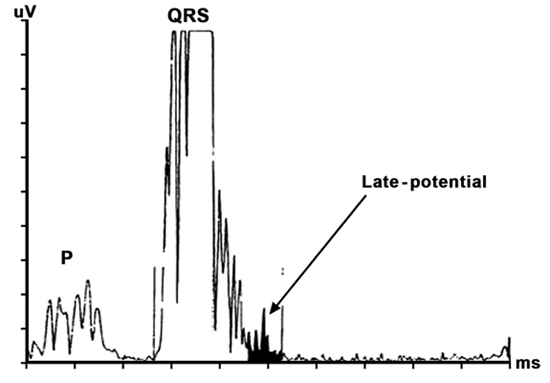
Figure 4.1. Example of a processed signal-averaged ECG revealing a high-frequency, low-amplitude LP.
Common criteria defining SAECG positivity include the following:
- QRS duration: The filtered QRS complex is longer than 114 ms (in absence of bundle branch block).
- Low-amplitude signal: The terminal filtered QRS complex remains below 40 microvolts for more than 38 ms.
- Root mean square: There is less than 20 microvolts of signal in the last 40 ms of the filtered QRS complex.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


