MECHANISM OF VENTRICULAR FIBRILLATION
Case presented by:
A 73-year-old white gentleman is admitted to the intensive care unit post-coronary artery bypass grafting and is having an uneventful recovery. He reports no chest pain, shortness of breath and is doing well, until he suddenly develops the rhythm shown in Figure 29.1. He is now pulseless and a code-blue is activated.
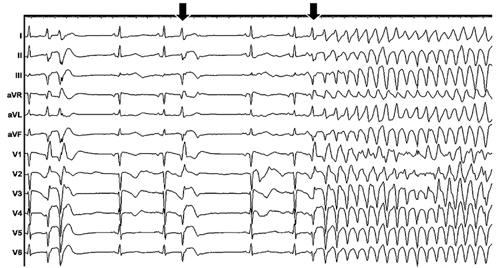
Figure 29.1. Clinical arrhythmia.
Question No. 1: What is the rhythm shown in Figure 29.1?
In Figure 29.1, the ECG tracing shows a sharp narrow premature ventricular complex (PVC), likely originating from the His-Purkinje system. The arrows show the similar PVCs that quickly degenerate into ventricular fibrillation (VF). VF is a ventricular tachyarrhythmia that has a constantly changing, disorganized-appearing morphology in the ECG.1 A multitude of data exists to support the fact that VF is not entirely “chaotic or disorganized.”2 Although the 12-lead surface ECG appears disorganized during VF, extensive endocardial and epicardial mapping have shown that dynamic patterns with variable degrees of organization maintain VF.2
There is no ventricular output during VF. Cardiopulmonary arrest ensues very rapidly in VF and immediate defibrillation with an electric shock is the only effective treatment. In a hospital setting, this can be accomplished very quickly. However, in the community, it takes several minutes for an emergency personnel to arrive and survival is low.
Question No. 2: Is there more than one type of VF?
There are multiple classifications of VF, although for practical purposes, the final treatment of VF does not differ for different types of VF, since an electric shock is the only definitive treatment. One classification of VF is “fine” and another is “coarse.” Coarse VF is frequently seen soon after the onset of VF (short-duration VF [SDVF]) and is associated with higher amplitude; hence it appears coarser on the surface ECG.3 As VF persists (long-duration VF [LDVF]), the amplitude decreases and “fine VF” is seen. However, it has been reported that coarse VF can be seen in some ECG leads simultaneously with fine VF in other ECG leads, suggesting that this classification is not always directly related to the underlying degree of organization of cardiac electrical activity during VF.
Two other classifications of VF are based on how it changes with time (Figure 29.2). Wiggers first elucidated the progression of VF by high-speed cinematography in canine hearts.4 He described 4 stages (see left side of Figure 29.2). Huang et al performed a cluster analysis of quantitative measurements of epicardial activation sequences obtained by recording simultaneously from over 500 electrodes in canine hearts (see right side of Figure 29.2).5 In the Wiggers’ classification, VF organization decreases continuously with time, while in the Huang classification, a brief period of increased organization occurs after about 1 minute of VF.
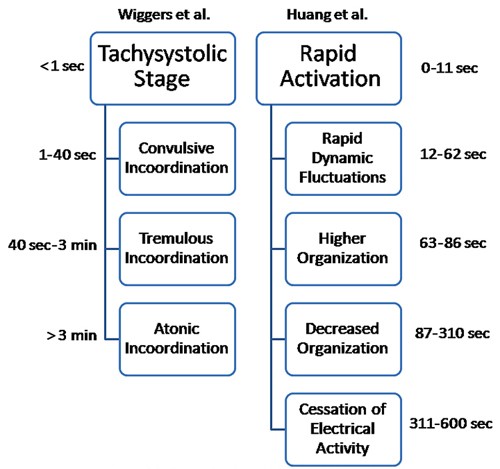
Figure 29.2. A schematic representation of the stages of VF, showing similarities in the stages as described by Wiggers et al4 and Huang et al.5
Another classification of VF in Types 1 and 2 is discussed in the answer to question no. 4.
Question No. 3: What are the common causes of VF?
The most common etiologies of VF are ischemic heart disease followed closely by heart failure.1 VF does occur in patients without structural heart disease as well, and idiopathic VF is now a well-known clinical entity.1 In idiopathic VF, certain inherited ion channelopathies can cause electrical instability.
Question No. 4: How is VF maintained?
There are 2 main theories on VF maintenance in humans: the multiple-wavelet hypothesis and the mother-rotor hypothesis.2
Multiple-wavelet hypothesis
This hypothesis was first proposed by Moe et al in 1964, to explain the activation patterns maintaining atrial fibrillation and was based on computer simulations.6 In the simulations, inhomogeneity in the refractory periods of cardiac muscle caused conduction block, which led to constantly changing reentry and multiple wandering wavelets of activation fronts. Some of these wavelets would be extinguished when they collided with other wavefronts or totally blocked in refractory tissue. Other wavelets would partially block, with the surviving fragments of activation fronts forming daughter wavelets that would continue to sustain VF. If enough wavelets partially blocked and reentered, fibrillation would be sustained. Therefore, all parts of the tissue participated in the maintenance of fibrillation. Later simulations have shown that such multiple wavelets could also be formed in completely homogenous tissue.
Mother-rotor hypothesis
This hypothesis was first proposed by Lewis in 1925, followed by Gurvich in 1975,7 and was more recently refined by Jalife and Pertsov.8,9
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


