HYBRID ABLATION FOR RECURRENT LEFT ATRIAL FIBRILLATION USING PERICARDIOSCOPY AND TRANSSEPTAL APPROACH
Case presented by:
A 68-year-old man presented with extreme symptoms of fatigue, palpitations, and dyspnea. He had a history of atrial tachyarrhythmias dating back to paroxysmal atrial fibrillation (AF) 11 years ago, and had been in persistent AF for the past 3 months. He failed 2 cardioversions 4 years ago, another cardioversion 7 months ago, and remained in AF despite sotalol.
The patient had a left atrial diameter of 5.7 cm. Left ventricular ejection fraction was normal, and there was no significant valvular disease, coronary artery disease, or underlying lung disease. Immediate preablation transesophageal echocardiogram (TEE) showed no left atrial appendage (LAA) thrombus.
Question No. 1: What are the advantages of pericardioscopic surgical ablation to treat AF?
A.It avoids painful sternal or intercostal incisions.
B.It is the least invasive surgical approach into the pericardial space currently available.
C.It should enhance the efficiency, efficacy, and safety of percutaneous ablation.
D.All of the above.
Discussion
Atrial fibrillation was present in approximately 3 million patients in 2005 and is responsible for more hospital admissions than any other heart rhythm disturbance in the United States.1 It is a leading cause of stroke, and may require lifelong anticoagulation in patients with significant risk factors.
Patients who are symptomatic despite the use of one or more antiarrhythmic drugs may be candidates for ablative therapy. Procedural success with ablation is influenced by factors such as left atrial size, duration since onset and persistence despite cardioversion. A multidisciplinary approach in which patients are seen by both an electrophysiologist and surgeon plays a critical role in the treatment of AF.
The patient described in this case underwent successful termination of persistent AF using a new hybrid ablation procedure2 that adds epicardial ablation via subxiphoid pericardioscopy to conventional mapping-guided transseptal catheter ablation.
The procedure was carried out in an electrophysiology/surgical hybrid suite that provided simultaneous multichannel intracardiac (using high right atrial and decapolar coronary sinus [CS] catheters) and surface electrocardiographic (ECG) monitoring; high-resolution fluoroscopy with 3D computed tomography (CT) registration of the left atrium, esophagus, and CS; CARTO electroanatomical mapping (Biosense Webster Inc., Diamond Bar, CA) with both intra-atrial and epicardial data points; and full surgical capabilities including laparoscopic monitors, carts, and instruments.
A 2-cm subxiphoid midline incision was made and an 11-mm port placed using Hassan technique for a 10-mm laparoscope. Two 5-mm accessory ports were placed for instruments. Reverse trendelenburg and CO2 insufflation provided visualization of the central tendon of the diaphragm. It was opened 2 cm to the left of the falciform ligament using conventional laparoscopic techniques. The pericardioscope cannula was inserted in the midline incision and guided to the oblique sinus under direct visualization with the aid of a 7-mm laparoscope. Ablation of the anterior left and right pulmonary vein (PV) trunks was also performed through this access.
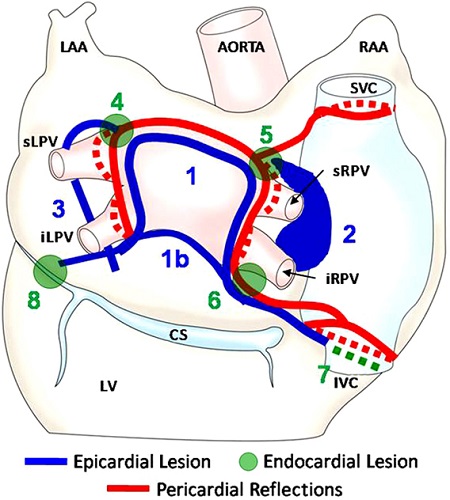
Epicardial radiofrequency ablation lesions were placed as shown in Figure 28.1, with epicardial and subsequent endocardial lesions highlighted. The 90-second lesions were placed using a flexible 3-cm saline-irrigated unipolar radiofrequency coil (Figure 28.2) that has the ability to attach to the atrium with vacuum. For curved lesions it can be placed on a guide wire rail that is built into the pericardioscope, or it can be used off the rail for more linear lesions. An esophageal temperature probe was placed with fluoroscopy guidance to monitor esophageal lumen temperature. Lesions are immediately terminated if the temperatures rise over 1°C.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


