TECHNIQUES OF SEGMENTAL PULMONARY VEIN ISOLATION AND CIRCUMFERENTIAL PULMONARY VEIN ABLATION DURING SINUS RHYTHM AND ATRIAL FIBRILLATION
Case presented by:
A ring catheter is placed in the left superior pulmonary vein (LSPV) during sinus rhythm in a patient presenting for his first ablation procedure for idiopathic paroxysmal atrial fibrillation (AF). During extrastimulus testing while pacing from the distal coronary sinus (CS) (Figure 23.1), 2 distinct potentials emerge on the ring-catheter recording.
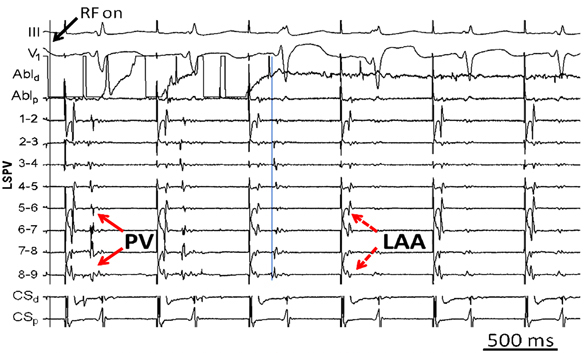
Figure 23.1. Coronary sinus pacing.
Question No. 1: Which of the following is true?
A.The pulmonary vein (PV) potential is recorded prior to the left atrial appendage (LAA) potential.
B.The LAA potential precedes the PV potential.
C.The first potential represents near-field activation of the left superior PV, while the second represents far-field contribution from the left inferior PV.
D.The 2 potentials are generated by a conduction delay into the LAA.
A.PV isolation followed by linear ablation at the left atrial roof.
B.PV isolation followed by ablation of complex, fractionated electrograms (EGMs).
C.PV isolation followed by linear ablation at the mitral isthmus.
D.PV isolation only.
E.Circumferential PV ablation with the end point of voltage abatement along the ipsilateral circular lesions.
A.Since the likelihood of esophageal injury is so low, proceed with left antral ablation without regard to the esophagus, using 30 watts of irrigated radiofrequency (RF) energy.
B.Proceed with left antral ablation using 35 watts of irrigated RF energy.
C.Complete the anterior aspect of the circumferential lesion, followed by RF energy delivery at the crux between the upper and lower PVs before targeting the posterior aspect near the esophagus using 25 watts of irrigated RF energy.
D.Perform left antral ablation using 35 watts of irrigated RF energy, guided by esophageal temperature monitoring.
E.Complete the anterior aspect of the circumferential lesion using RF energy, followed by cryoballoon ablation of the left-sided PVs.
A.Perform right superior PV isolation using a 28-mm cryoballoon instead of conventional RF energy since the risk of phrenic nerve injury with the former is lower.
B.Since the risk of phrenic nerve injury is low, and reversible in many cases, proceed with ablation without further testing.
C.Withdraw the ablation catheter slightly more proximally, and repeat high-output pacing. If negative, then proceed with ablation.
D.Abort the procedure, and refer the patient for a surgical ablation procedure.
E.Forgo isolation of the right superior PV, and perform linear ablation at the left atrial roof instead to minimize the risk of AF recurrence.
A.Perform complete circumferential ablation around both antra with the end point of PV isolation.
B.Since the patient has failed a PV-based procedure, perform linear ablation at the roof and mitral isthmus with demonstration of conduction block.
C.Map and ablate the discrete PV breakthroughs rather than recreating the entire circumferential lesion set.
D.Administer isoproterenol to determine which of the 2 reconnected PVs is arrhythmogenic, then isolate the culprit PV.
E.Induce AF with rapid atrial pacing, then target complex fractionated EGMs with the end point of AF termination.
Discussion
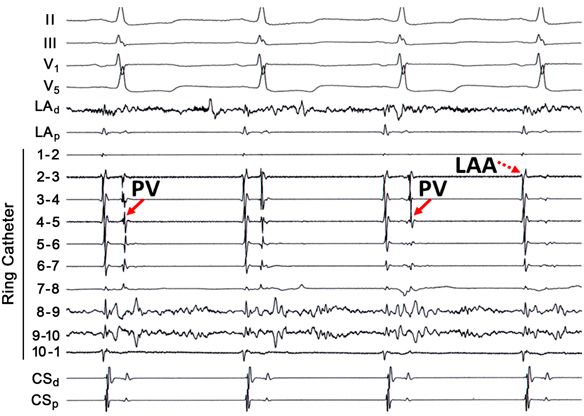
Despite the promulgation of various ablation techniques for AF over the last decade, pulmonary vein remains the cornerstone for patients with both paroxysmal and persistent AF. The original description of elimination of PV triggers involved RF energy delivery within the culprit vein. Because ablation within a vein may be complicated by stenosis, ostial ablation of the PVs became the preferred method of PV isolation. Ostial or segmental PV isolation involves identifying PV breakthroughs on a circular mapping catheter placed at the PV ostium. Although PV potentials may be recorded on all bipoles of the circular catheter, identification of the earliest activation may obviate RF energy delivery around the entire circumference of the PV ostium (Figure 23.2). This may have important implications for procedure times and risk of complications, such as PV stenosis. However, in some patients the left atrial (LA)-PV connections may be quite broad, requiring ablation around much of the ostium.
Discrete PV breakthroughs may be identified during sinus rhythm and atrial pacing. In a patient who has not previously undergone PV isolation, the atrial and venous potentials frequently appear fused on the circular mapping catheter. Atrial pacing, typically from the CS or the LA appendage, advances the atrial component, unmasking the PV potentials. The earliest activation is then identified and then targeted for ablation. After elimination of a PV breakthrough, the activation pattern frequently changes, suggesting the existence of multiple LA–PV connections. The end point is absence or dissociation of PV potentials at the ostium. Although some patients may only require isolation of only the “culprit” vein, that is, one that has been shown to be arrhythmogenic on provocative testing, it is reasonable to electrically isolate all PVs to prevent recurrent arrhythmias.
Pacing from the LA appendage or the CS is not as helpful in discriminating between atrial and venous potentials on tracings from the right-sided PVs. For the right superior PV, one can employ activation mapping with respect to the P wave on the ECG to determine if the residual potential is of atrial or venous origin. If the EGM in question occurs within 30 ms of the onset of the P wave, then it is very likely due to far-field activation of the superior vena cava (SVC).1 If the EGM is advanced to the stimulus artifact during right atrial pacing, a far-field origin is confirmed (Figure 23.3). For the right inferior PV, posterior LA pacing may be helpful in discriminating between far-field LA potentials and near-field PV potentials.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


