TARGETING COMPLEX FRACTIONATED ATRIAL ELECTROGRAMS TO ELIMINATE ATRIAL FIBRILLATION
Case presented by:
A 55-year-old man with persistent atrial fibrillation (AF) was first diagnosed 4 years prior to presentation. He has failed to maintain sinus rhythm despite treatment with propafenone and subsequently dofetilide and 3 cardioversions. Atrial fibrillation recurred within a week after each cardioversion and has been persistent over the last 18 months. He has fatigue, exertional dyspnea, and decreased exercise tolerance. His mean ventricular rate at rest is 78 bpm on a combination of metoprolol and diltiazem. His left atrial diameter is 48 mm on transthoracic echocardiography and ejection fraction is 0.50. He has a history of hypertension. After having discussed the treatment options, he expressed his interest to proceed with radiofrequency catheter ablation to eliminate AF.
During percutaneous radiofrequency catheter ablation, antral pulmonary vein (PV) isolation was performed and all PVs were electrically isolated. Linear ablation along the left atrial roof and mitral isthmus was also performed. Because AF still persisted, complex fractionated atrial electrograms (CFAEs) were targeted and resulted in termination of AF to sinus rhythm (Figure 22.1).
Question No. 1: What are the potential mechanisms of AF that may contribute to CFAEs?
A.High-frequency sources with rapid activation rates (rotors).
B.Wavebreak and fibrillatory conduction.
C.Discordant activations from multiple layers of atrial myocardium and anisotropic conduction.
D.Shortened effective refractory period (ERP) at sites of autonomic innervation enabling rapid activation rates.
E.All of the above.
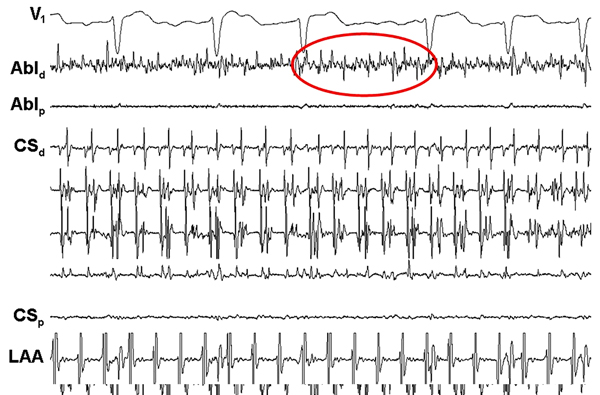
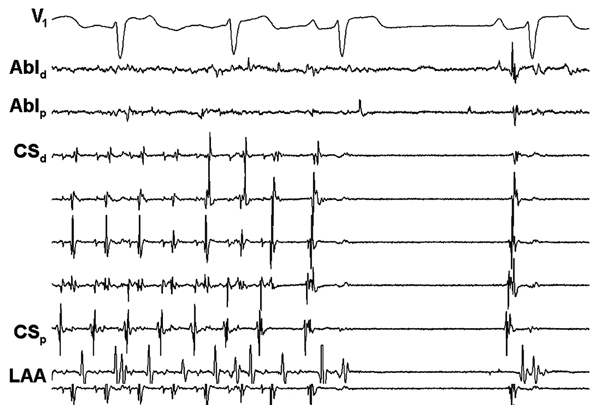
Discussion
In patients with paroxysmal AF, the mechanisms of AF primarily depend on PV arrhythmogenicity.1,2 Therefore, antral PV isolation is usually sufficient to maintain sinus rhythm in 75% to 85% of patients with paroxysmal AF.3–6 The vast majority of recurrences appear to be due to recovery of conduction into the PVs or due to arrhythmogenic foci in the other thoracic veins. However, in patients with persistent AF, extensive left or biatrial electroanatomical remodeling occurs, and the atrial tissue becomes capable of maintaining AF independent of the PVs. Therefore in these patients, antral PV isolation often has modest efficacy despite repeat ablation procedures that usually address PV reconnection.
To date, 4 major mechanisms have been suggested to play a role in the genesis of AF: (1) multiple, randomly wandering wavelets, ever changing in number and direction, which result in total disorganization of activity. This hypothesis was proposed to explain perpetuation of AF based on a computational model that was developed almost 50 years ago;7 (2) high-frequency reentrant sources (rotors) generating spiral waves that interact with functional or structural heterogeneities in the atria, with resultant spatially distributed wavebreaks and fibrillatory conduction;8–10 (3) thoracic vein hypothesis;1,11 and (4) autonomic modulation with parasympathetic versus sympathetic imbalance that results in a decrease in atrial ERP, increased automaticity, and facilitated triggered activity that promotes premature atrial depolarizations and fast activation rates.12
To improve the efficacy of catheter ablation in patients with persistent AF, strategies to target and eliminate the residual mechanisms of AF beyond the PVs have been proposed. Linear ablation is often performed between well-defined anatomical landmarks such as along the left atrial roof between the left and right superior PVs, or along the mitral isthmus, or between the left inferior PV and lateral mitral annulus.13–16 Lines at other sites such as the anterior left atrial wall, septum of the left atrium between the right superior or inferior PV, and mitral annulus have also been performed. Linear ablation may improve the efficacy of PV isolation by a variety of mechanisms: (1) linear ablation may partition the left atrium similar to a classic maze procedure so that the maximum wavelength that can be accommodated is shorter than the wavelength of the clinical reentrant circuit; (2) linear ablation includes sites where high-frequency sources may be more prevalent such as the left atrial roof; and (3) linear ablation may directly intercept the course of a macroreentrant circuit.
Few reports suggested a potentially beneficial role of linear ablation after antral PV isolation in patients with persistent AF. However, a previous report that utilized PV isolation, linear ablation, and ablation of CFAEs in a randomized order demonstrated that all 4 steps were necessary in >80% of the patients with long-standing persistent AF to terminate AF.17 Linear ablation was often necessary to terminate AF after ablation of CFAEs.14 During linear ablation, complete conduction block across the ablation line must be achieved and sought after as an end point.13,18 Otherwise, linear ablation with incomplete conduction block increases the probability of proarrhythmia and has been associated with a higher prevalence of atrial flutter after the ablation procedure. Given the occasional challenge in achieving complete conduction block, particularly along the mitral isthmus, and potential risk of complications due to excess ablation (eg, coronary artery injury), linear ablation is often not the preferred first choice unless AF converts to a macroreentrant atrial flutter in contemporary practice. However, the precise role of linear ablation versus ablation of CFAEs as the next step after antral PV isolation remains to be determined.
A recent study that targeted CFAEs as the sole ablation strategy in patients with paroxysmal and persistent AF reported good clinical outcomes and rejuvenated the interest in CFAEs.19 Based on analysis of epicardial unipolar EGMs recorded during AF, CFAEs have been suggested to indicate sites of slow conduction or conduction block, pivot points for reentrant circuits, and wavefront collision (Figure 22.2).20 Therefore, sites of CFAEs may indicate critical parts of reentrant circuits, and ablation of these sites may interfere with the reentrant mechanisms of AF. On the other hand, CFAEs may actually reflect fibrillatory conduction of broken spiraling wavefronts emanating from a nearby-high-frequency rotor.21,22
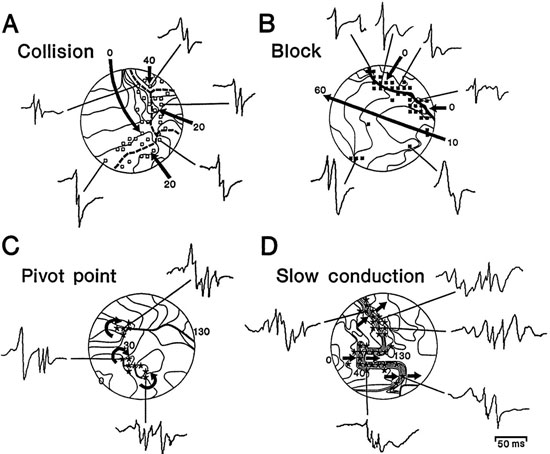
Figure 22.2. Complex EGMs. Isochronal maps of the unipolar EGMs acquired during epicardial mapping of the human atrium during AF demonstrated the role these EGMs may play in reentrant arrhythmias. See text for details (Adapted from Konings et al9 with permission from Wolters Kluwer Health).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


