SYNCOPE AND SUSPECTED SINUS NODE DYSFUNCTION
Case presented by:
Mr. R is a 57-year-old gentleman seen in consultation regarding sinus bradycardia noted during evaluation for a minor surgical procedure.
Mr. R is employed as an airline pilot safety instructor. He reports one episode of loss of consciousness 3 years ago. After standing for a prolonged period of time he began to feel warm, diaphoretic, and nauseated. The next thing he remembers is waking up on the floor with co-workers asking him if he was okay. He was told he was “pale as a ghost.” There was no incontinence and no injuries. After the event he felt fatigued but reports no other sequelae. His co-workers wanted to call an ambulance but he declined. He rested for several minutes, drank some water, and returned to work. He denies any other syncopal events. He reports no symptoms of near syncope.
Mr. R is physically fit. He runs on a daily basis and lifts weights 3 times a week. He reports no family history of cardiac disease or arrhythmia. He has no significant past medical or surgical history, other than obstructive sleep apnea. He has been fitted for, and wears, a CPAP mask. He takes no medications and uses no over-the-counter supplements. His physical examination, other than a resting heart rate of 50 bpm, is entirely normal. His blood pressure was 116/68 while at rest, seated.
Prior to electrophysiology (EP) consultation, an echocardiogram demonstrated a structurally normal heart. A 24-hour Holter monitor showed an average heart rate of 67 bpm. The maximum heart rate recorded was sinus at 152 bpm and was recorded while he was running on a treadmill. The minimum heart rate was 44 bpm recorded at 02:00. At that time, first-degree atrioventricular (AV) block was noted. No higher degree block was recorded. No ventricular ectopy was recorded. Rare atrial premature beats were seen with no reported associated symptoms. There were no pauses > 2 seconds.
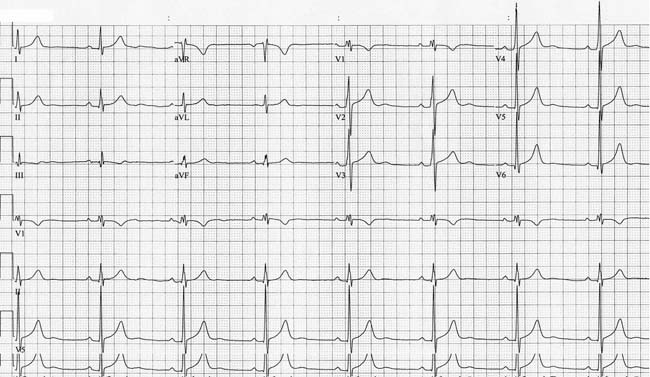
Figure 2.1. Baseline ECG obtained during preoperative evaluation.
Question No. 1: As a consultant, you would recommend which of the following?
A.A tilt table study.
B.A 30-day event recorder (looping).
C.An implantable loop recorder (ILR).
D.An exercise study.
E.No further evaluation.
Discussion
Review of the 12-lead ECGobtained during the patient’s preoperative evaluation (Figure 2.1) shows a P-wave morphology and axis that are consistent with a sinus node origin at a rate of 47 bpm. The PR interval is normal at 180 ms; ST segment, T waves, and QT intervals are normal.
No pathologic Q waves are seen. The QRS duration and frontal axis are both normal. The QRS transition in the precordial leads is early. While this ECG finding raises the question of posterior infarction or right ventricular (RV) hypertrophy, an echocardiogram obtained prior to consultation demonstrated normal RV size with no wall motion abnormalities seen. In this case the early precordial transition noted is a normal variant.
Careful analysis of the QRS morphology demonstrates notching in the limb and a slurring in the upstroke of the QRS in the right precordial leads, suggesting mild nonspecific intraventricular conduction delay. This has not resulted in prolongation of the QRS. The slurring in V1 and V2 should not be confused with ventricular preexcitation. U waves are noted in the midprecordial leads. U waves can be seen in patients with hypokalemia, but Mr. R’s chemistry evaluation was entirely normal.
It is difficult to formulate an efficient evaluation strategy without a clear understanding of the clinical question being asked. In this case we are being asked whether or not Mr. R’s sinus bradycardia is pathologic, how does it relate to his earlier syncopal episode and does he need a pacemaker? In short, does Mr. R have sinus node dysfunction (SND)? SND can be broadly defined as a heart rate that is inappropriate for the physiological needs of the body. The incidence of SND increases with aging, but it has been described in all age groups. In the United States, SND is the indication for roughly half of all pacemaker implantations. In the face of an aging population, diagnosis and treatment of SND will consume an increasing proportion of health care resources. The need for an efficient and cost-effective approach to this syndrome is obvious.
Anatomy and Structure of the Sinus Node
Shortly after the turn of the last century Arthur Keith, then working on the question of whether the caval openings closed during contraction of the right atrium, noted in human hearts “a small condensed area of tissue, just where the cava sank into the auricle.” A few years later, after learning of Tarawa’s description of a “muscular” connection between the atria and the ventricles, Keith enlisted the aid of Martin Flack, a medical student at the time, in examining the hearts of a variety of animals for the structure described by Tarawa. When Flack described a structure he had identified in the right atrium of a mole Keith recognized this as being similar to the “condensed area” he had earlier identified in human hearts. They examined the remaining specimens and found a similar structure in each. In 1907, Keith and Flack published their paper describing what would later be referred to as the “sinus node.”
The sinus node is located along the superior portion of the crista terminalis (CT) near the lateral aspect of the junction of the superior vena cava (SVC) and right atrium. However, the sinus node is not a small dot or sphere as often depicted in cartoons of the conduction system. In some mammals, the node extends along the entire intracaval region. In humans, histologically, the sinus node lies in the subepicardium and extends along the superior portion of the CT. The predominant pacemaking region is generally located in the superior portion of the CT. At lower heart rates the predominant pacemaking region shifts to more inferior locations along the CT. This shift in the location of the predominant pacemaking region may be large enough to result in a change in the P-wave axis on the surface ECG. Distinguishing whether a slow atrial rhythm, with a superiorly directed P-wave axis, is truly an ectopic atrial focus versus sinus bradycardia originating from the inferior aspect of the CT can be difficult on an isolated ECG. The presence of sinus node-like capability throughout the CT may explain the frequency with which focal atrial tachycardias arising in the right atrium are localized to the CT.
Histologically and functionally the sinus node is a complex structure, a structure of which basic EP is still debated despite a century of study. The mechanism(s) by which the pacemaking activity of the sinus node is regulated remains an area of controversy. Similarly, the question of how a small mass of cells is able to both electrically capture the much larger mass of the atrium and concomitantly protect itself from suppression by the larger and more hyperpolarized atrial myocardium remains incompletely understood. A great deal is known regarding details of ion channel expression, ionic currents, and electrical coupling within various regions of the sinus node but the larger question of how these components interact remains to be elucidated.
The primary pacemaking cells of the sinus node lie in the center of the sinus node. Cells in this region demonstrate typical Phase 4 depolarization that is characteristic of cells that demonstrate automaticity. As in other excitable tissues once a certain voltage threshold is reached an action potential is generated (Figure 2.2).
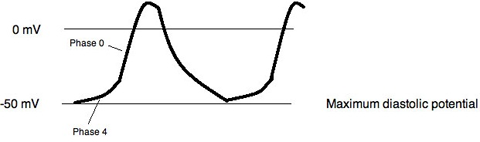
Figure 2.2. Sinus node action potential.
The upstroke of the action potential recorded from the center of the sinus node is slow due to the paucity of a Na current, INa. In these cells the primary current resulting in depolarization is the L-type Ca2+, ICa,L. Other inward currents involved in the pacemaker action potential include, T-type Ca2+, ICa,T and the so-called “funny” current, If. A selective blocker of If, ivabradine is now available as a heart rate lowering agent. The Na2+-Ca2+ exchanger, responsible for Ca2+ extrusion from the cell is electrogenic resulting in a net inward current, INaCa. This current is not insignificant, equaling roughly half of the amount of current carried by ICa,L and ICa,T. Occurring during diastole INaCa may play a role in pacemaking. The role that Ca2+ release from the sarcoplasmic reticulum plays in the pacemaking activity of the sinus node remains uncertain.3,4
In myocardium the inward rectifying K+ current, IK,I, is primarily responsible for maintaining a stable diastolic resting potential, therefore it should not be surprising that this current is absent in pacemaking cells. The rapid and slow delayed rectifier K+ currents, IK,r and IK,s, are present helping to repolarize the cell and establishing the maximum diastolic potential.3 The primary outward and inward currents and the ion channels carrying those currents in the central pacemaking cells of the sinus node are summarized in Table 2.1.
Table 2.1. Principle Currents Involved in the Sinus Node Action Potential
| Outward currents (primary ion channel) | Inward currents (primary ion channel) |
| ICa,L (Cav 1.3) | IK,r (ERG) |
| ICa,T (Cav 3.1 and Cav 3.2) | IK,s (K,LQT1) |
| If (HCN4, HCN1, and HCN2 also present) | |
| INaCa |
Once a pacemaker action potential has been generated it must then be conducted out of the sinus node if the atrium is to be activated. As discussed above, how the sinus node is able to accomplish this task yet also protect itself from suppression by the mass of atrial myocardium remains unresolved. There is a gradual change in cell type and cell morphology as one moves outward from the center of the sinus node. There is no clearly defined border between the node and the myocardium. Unlike working myocardium electrical coupling in the sinus node is poor and cell-to-cell conduction is slow. This is one of the likely mechanisms that protects the sinus node from suppression by the more polarized atrial tissue surrounding it. The poor electrical coupling found in the sinus node is related to a change in the connexin makeup of the gap junctions. This poor electrical coupling and slow conduction would make it difficult for the sinus node to capture the atrium suggesting that in the periphery electrical coupling should be stronger and more rapid. In the sinus node of the rabbit there is evidence that this is the case, with cells in the periphery expressing different connexins than cells in the central portion of the sinus node.1
A more detailed description of the structure and function of the sinoatrial (SA) node is beyond the scope of this chapter. Interested readers are referred to a recent review of this topic by Dobrzynski and colleagues.
SND can present in several ways—persistent inappropriate bradycardia, sinus pauses, atrial fibrillation (often with a slow ventricular response) or a combination of intermittent bradycardia and atrial tachyarrhythmias (the so-called bradycardia–tachycardia syndrome [BTS]). The tachyarrhythmia most commonly seen is atrial fibrillation, but atrial flutter and other reentrant or focal atrial tachycardias also occur. BTS is the most frequent manifestation of SND and is also the SND form with the highest incidence of syncope. Syncope most often results from the prolonged sinus pauses following the cessation of a paroxysmal supraventricular tachyarrhythmia. Sinus node pauses can also be seen without a preceding tachyarrhythmia. If prolonged, these pauses may also result in syncope or the feeling of impending syncope.
The monitor strips in Figure 2.3 are typical of BTS. The strips are continuous and demonstrate an episode of paroxysmal atrial fibrillation with a rapid ventricular rate. When the atrial fibrillation terminates, a 5.2-second pause follows. Although the episode occurs at 03:24 in the morning, this particular patient was awake, having been awoke by palpitations. The recorded pause was associated with this individual’s feeling of impending collapse; permanent pacing was recommended.
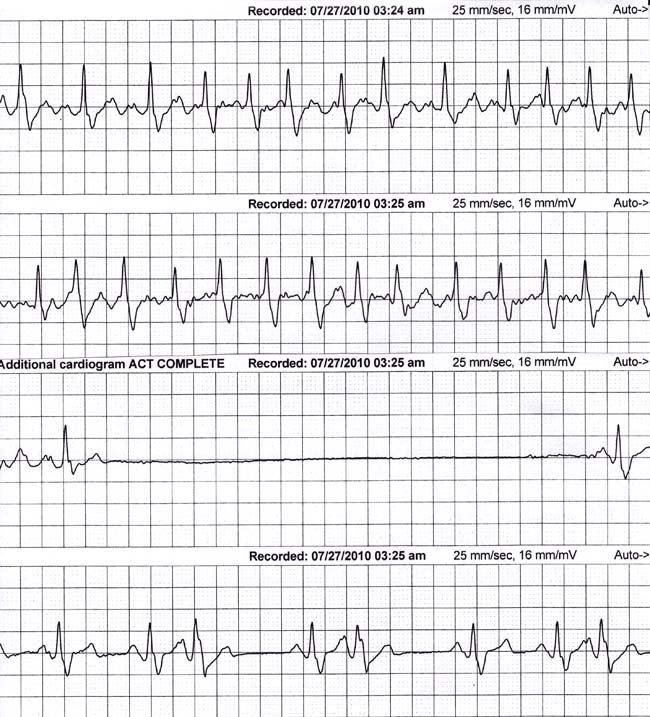
Figure 2.3. Bradycardia–tachycardia syndrome (BTS).
The prolonged pauses that occur either spontaneously or after termination of a supraventricular tachycardia illustrate another facet of SND that is often overlooked, namely that this entity encompasses more than the sinus node alone. During such pauses one of the heart’s subsidiary pacemakers should have become manifest. The absence of an “escape” rhythm suggests a more diffuse abnormality affecting the function of all cardiac pacemaker foci.
Persistent or inappropriate sinus bradycardia is rarely the cause of syncope. When SND presents in this fashion, symptoms are more often generalized fatigue or exercise intolerance. Patients frequently attribute such symptoms as a sign they are “getting old”. Determining to what extent an older patient’s declining exercise ability and fatigue is normal aging versus pathology is difficult.
In Mr. R’s case, his ECG is borderline abnormal by definition; the heart rate is less than 60 bpm. The definition of what is and what is not a normal heart rate is arbitrary, a historical fait accompli. If SND were to be diagnosed on that basis alone many of the readers of this text, as well as most very fit citizens, would be considered to have SND. More than a heart rate is needed to determine the significance of sinus bradycardia in a particular individual. Is the sinus bradycardia associated with symptoms? Is the sinus rate inappropriate for the level of physical activity? Can the sinus bradycardia be attributed to other causes? Does the sinus bradycardia occur in the setting of other atrial tachyarrhythmias such as atrial fibrillation?
In the evaluation of bradycardia, the presence or absence of symptoms is of paramount importance. If a patient’s symptoms can clearly be attributed to a bradyarrhythmia, no further diagnostic evaluation is needed. At that point the only pertinent question is what can be done to address the bradyarrhythmia.
Does Mr. R have symptoms? No. He reports feeling well. He has had no change in his exercise capacity. He is not fatigued. He does not have palpitations. He denies feeling unsteady, dizzy, or lightheaded. He states he feels “perfect”. What about his report of a syncopal episode? In fact, the description of that episode was classic for a vasovagal faint.
What evaluation, if any, is required in patients with asymptomatic sinus bradycardia? In general such patients, if truly asymptomatic, do not require evaluation. In Mr. R’s case the need for surgery has raised concerns about the safety of surgery in the face of his sinus bradycardia. Granted he is asymptomatic but does he have SND? Is his heart rate in the 40s at rest because he is an aerobically fit man with high parasympathetic tone at rest or is his sinus node abnormal?
The sinus node is extensively innervated by both the parasympathetic and sympathetic nervous systems. Aerobically fit people have relative sinus bradycardia because at rest the parasympathetic nervous system input predominates. However, sinus bradycardia may be the result of intrinsic SND, or extrinsic factors (often termed “extrinsic” SND), particularly drugs. In any case, in health, the sinus node should respond normally to changes in autonomic tone. A withdrawal of parasympathetic tone or an increase in sympathetic tone will result in an increase in heart rate. How the sinus node responds to alteration in autonomic tone is critical in determining whether sinus bradycardia is a clinically important problem in a particular individual.
There are several methods of altering autonomic tone and studying its effects on sinus node function. The simplest test of this type is exercise. Physical activity results in withdrawal of parasympathetic tone and an increase in sympathetic outflow. The combined effect is an increase in the heart rate. Failure of the heart rate to increase appropriately in the face of increased metabolic demand is how we defined SND. If the inability to increase the heart rate appropriately is the primary manifestation of SND, the condition is referred to as “chronotropic incompetence”. Ordering an exercise test was one possible answer in the question posed above. An exercise study allows a direct assessment of the ability of the sinus node to respond to physiological stress. In this case an exercise study is not needed. Mr. R underwent Holter monitoring that demonstrated a normal heart rate response to exercise making an exercise test unnecessary.
When a Holter monitor is used to evaluate heart rate response, patients must understand the importance of continuing to perform their usual activities and recording those activities in the diary provided. If the patient remains sedentary during the recording period, the Holter will tell us what we already know—the heart rate is low at rest. In sedentary patients, Holter monitoring often leads to a “the chicken or the egg” dilemma. Does the Holter demonstrate a low maximum heart rate because the patient is sedentary or is the patient sedentary because of the low heart rate? In these patients an exercise test is more useful.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


