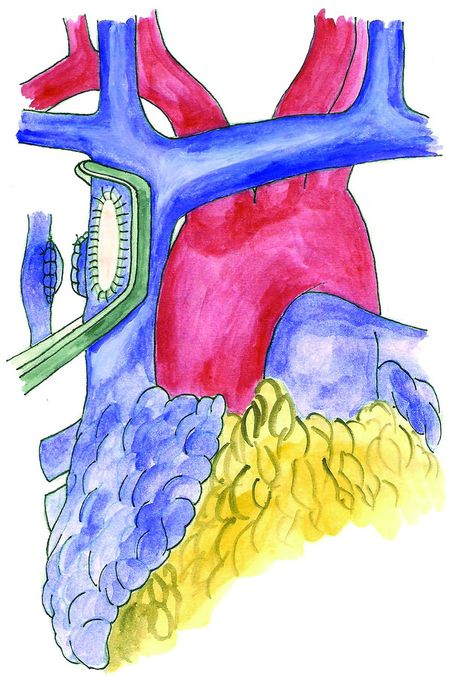
Anatomy of the superior vena cava and brachiocephalic veins.
The junction between the SVC and the right atrium lies within the pericardium that surrounds the anterolateral surface of the vessel for about 2 cm. At the level of the anterolateral aspect of the junction with the atrium is located the sinus node.
Anatomy of the collateral SVC routes:
A) Azygous venous system: it drains directly into the extrapericardial posterior aspect of the SVC immediately above the level where the right pulmonary artery crosses the vein posteriorly.
B) Internal thoracic venous system: through this system blood converges into the inferior vena cava (IVC) from the internal thoracic vein through the epigastric veins and the external and common iliac veins.
C) Vertebral venous system: The blood flows into the IVC through the brachiocephalic veins and the intercostal, lumbar, and sacral veins.
Clinical presentation
Obstruction of the SVC due to compression, invasion, constriction, or thrombosis shows a different pattern of clinical presentation based on its acuity and degree. The SVC drains blood from the head, neck, and upper extremities. If the SVC obstruction progresses slowly, adequate collateral drainage develops, and patients potentially show no or mild symptoms. On the other hand, if vascular obstruction develops acutely, patients are obviously more symptomatic since the collateral circulation has no time to develop and compensate. In this case the signs and symptoms might be dramatic. The eyes are usually affected first, and patients complain of tearing and swelling of eyelids. They also refer to headache, dizziness, tinnitus, and a bursting sensation in the head exacerbated when bending forward. The face, neck, and arms become red and edematous; the superficial veins of the chest may be distended if they are part of the collateral circulation. These symptoms are greatly increased when also the azygous vein is obstructed. Some patients may find some symptomatic relief in the upright position; for this reason, they sleep in a chair to avoid dyspnea. The venous hypertension might cause life-threatening complications such as cerebral edema, thrombosis, and hemorrhage or laryngeal and/or glossal edema.
Since most of the SVC obstructions are caused by bronchogenic carcinoma or mediastinal neoplasms, patients might show also symptoms related to the tumor, including paraneoplastic syndromes.
Pathophysiology
The SVC is located in an anatomic compartment that has limited distensibility; due to the thin walls and inner low pressure it is easily obstructed by compression, invasion, and constriction; also, thrombosis due to hypercoagulation, intimal damage, and/or stasis may be involved. Severe obstruction dramatically increases the endovascular pressure up to 400–500 cmH2O. As aforementioned, in case of acute SVC obstruction, the collateral systems do not have time to accommodate the increased blood flow. On the other hand, in case of slow progression of obstruction, palliation from collateral circulation is more pronounced. The site of obstruction is extremely important: when the azygous vein orifice is not involved, the collateral pathways are more efficient since this system easily accommodates the shunted blood with acceptable flow due to its caliber and distensibility. On the other hand, when the azygous system can’t compensate due to the location of the tumor, the blood flow runs through the other venous systems that are less efficient for the smaller caliber and major length of the pathway; for these reasons, in these situations, signs and symptoms are more pronounced.
Diagnosis
SVC syndrome is a clinical diagnosis; radiologic confirmation is usually not strictly necessary, although it might be helpful. However, it is crucial to diagnose the underlying disease since treatment is different according to its nature.
Routine radiologic studies include chest X-ray, computed tomography (CT), and magnetic resonance imaging (MRI). Also Doppler studies[23] and digital subtraction angiography have been employed[24].
SVC syndrome is rarely a true emergency[25]; for this reason, any treatment should be delayed until a precise histologic diagnosis is obtained. However, if urgent symptomatic treatment is deemed necessary, the diagnosis can be delayed until at least endovascular treatment is performed, especially in case of inoperable tumors.
The approach to obtain tissue for diagnosis depends on tumor location, clinical status of the patient, and available expertise. According to these key points, bronchoscopy, needle biopsy, endoscopic ultrasound (EUS) or endobronchial ultrasound (EBUS)[26], mediastinoscopy[27], anterior mediastinotomy, median sternotomy, video-assisted thoracoscopy (VATS), and even thoracotomy might be indicated.
Treatment
Management depends on etiology and severity of symptoms. It should take into account several key points. Measures such as head elevation, fluid restriction, administration of diuretics, and supplemental oxygen are useful until the diagnosis is obtained and treatment can be planned.
According to the clinical presentation and to histologic diagnosis, pharmacologic treatment, radiation therapy (with or without concomitant chemotherapy), and invasive procedures including endovascular stent deployment and surgical resection and reconstruction should be considered.
Patients affected by catheter-related SVC thrombosis should receive anticoagulant treatment and may require catheter removal. Heparin administration favors clot lysis; as an alternative, thrombolytic agents can be given through the catheter itself, especially if it is centered in the thrombus. This therapy is extremely successful when started within 48 hours of the onset of thrombosis; its efficacy strongly decreases after a longer period of time. Urokinase is usually more successful than streptokinase[28]. In patients with extensive malignancy, this treatment should be evaluated more carefully.
In patients with SVC syndrome related to inoperable tumors, radiotherapy is an effective treatment modality, and it is usually the initial choice once the diagnosis is available. Many fractional dose schemes have been proposed; however, despite the various dosing regimens described, most patients receive a total of 20 to 30 Gy[29,30].
Percutaneous delivery of metallic stents has become a viable option to treat patients with SVC obstruction not suitable for surgical resection. Several endovascular stents are available basically in two designs: self-expanding and expandable[31,32]; however, the Wallstent (Boston Scientific, Natick, MA) is certainly the most commonly used[33]. Stents are usually employed in association with adjuvant therapies: intravenous heparin is administered before and after placement[34]; thrombolysis is performed if clots occlude the lumen of the vessel.
Surgery is overall rarely indicated; however, it plays clearly an important role in patients with resectable lung cancer or mediastinal tumors without distant or lymphatic spread. Notwithstanding that, resection and reconstruction of the SVC are still considered major technical and anesthesiological challenges, clear benefits have been reported with adequate patients selection, planning of the surgical strategy, and prevention of complications[35–37].
Median sternotomy is the optimal approach for anterior mediastinal tumors, while right thoracotomy in the fourth or fifth intercostal space should be employed for upper lobe tumors invading the SVC. These approaches allow optimal exposure and access to the lung, SVC and azygous vein, trachea, pulmonary hilum, and right atrium; the left brachiocephalic vein is obviously more visible through median sternotomy. Cardiopulmonary bypass (CPBP) and shunts are easily instituted through both approaches when required.
Intraoperative management is extremely important and should be planned in advance. Partial caval clamping (tangential) or clamping of a vessel with a long history of occlusion and presence of a viable collateral circulation is usually well tolerated. In these situations, the duration of clamping is not a major issue. On the other hand, complete clamping of an unobstructed vessel causes an important hemodynamic derangement with consequent venous pressure increase, decrease in arterial pressure, and reduced brain arteriovenous gradient. This might cause brain edema, hemorrhage, or transient dysfunction. The hemodynamic imbalance is reduced by an aggressive intraoperative management; fluid implementation by macromolecules, blood, and plasma is recommended and should be obtained through a femoral vein access; diuretics should be administered to reduce brain edema; anticoagulation therapy is mandatory during and immediately after the operation. Intravascular or extravascular shunts might be useful when a prolonged complete clamping time is required; CPBP is rarely required.
If the SVC is involved for less than 30% of its circumference, partial resection is usually feasible, and reconstruction can be performed either by direct suture of the tangential resection or by interposition of a patch (Figure 15.2); in this case, autologous material (either pericardial or venous) can be successfully employed. When a more extended involvement of the vessel is present, complete resection en bloc with the primary tumor and prosthetic reconstruction are required. Reconstruction can be accomplished by SVC replacement if the confluence of the brachiocephalic veins is disease-free (Figure 15.3). To avoid kinking of the prosthesis, whatever material is used, the length of the conduit should be adapted to keep it under moderate tension; this is particularly important when the operation is carried out through a median sternotomy, since approximation of the bone edges may cause kinking of the conduit. If the confluence of the innominate veins is involved by the tumor, the reconstruction is usually performed connecting only the two veins to the inferior SVC stump or to the right atrium, closing the contralateral vessel (Figure 15.4). Revascularization of both innominate veins connected independently to the right atrium is usually not performed for the risk of thrombosis, unless the collateral circulation in the neck is completely absent (previous neck surgery or radiotherapy). However, complete resections of the SVC and both brachiocephalic veins with a reconstruction achieved by a Y-shaped synthetic conduit have been reported[38].
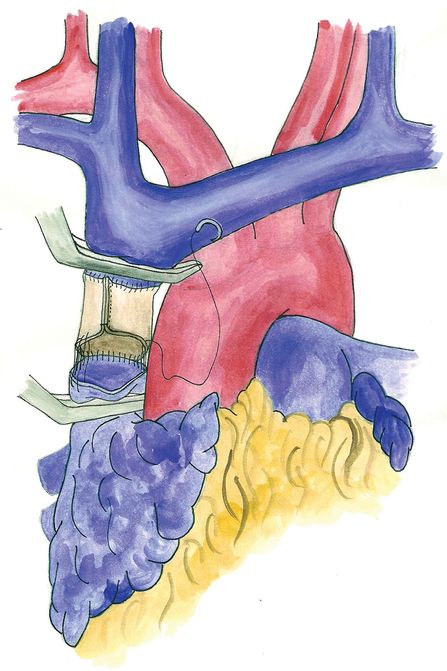
Superior vena cava reconstruction with a bovine pericardium conduit.
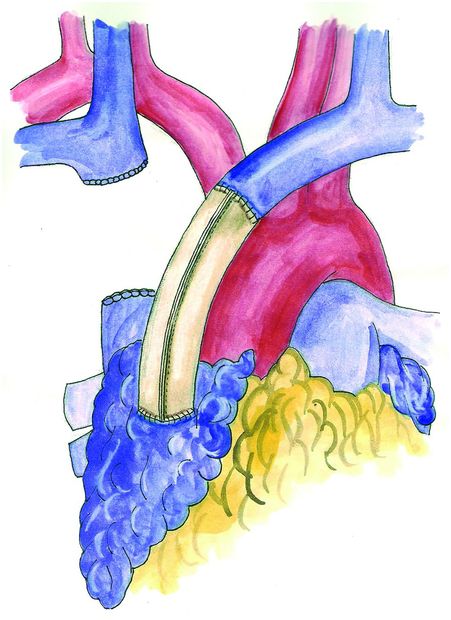
Revascularization between the left brachiocephalic vein and the right atrium with a conduit of bovine pericardium.
Both biologic (autologous and heterologous) and synthetic materials are nowadays available for vascular reconstructions. Biologic materials include autologous or bovine pericardium, azygous vein, and saphenous vein; they clearly show improved biocompatibility, decreased risk of infectious complications and thrombosis, and lower costs. Autologous pericardium has been used especially for patch reconstruction. It shows several advantages: it has adequate thickness and resistance; it is cost-free and available on both sides of the chest; it also offers a larger amount of tissue when compared with venous patches. An original method of fixation of autologous pericardium with glutaraldeyde[39] has been described to further improve the technical features of this material; with this technique, the fixed leaflet becomes stiffer with a reduced tendency to shrink or curl, and it is easier to tailor and suture.
Bovine pericardium is certainly the most used heterologous biologic material. It shows stiff edges and a limited tendency to retract; it is more frequently used for complete SVC replacement because autologous pericardium is not sufficient to create a long conduit. In our experience, the use of this material does not require long-term anticoagulation. An original technique has been described to construct a pericardial conduit suitable for SVC reconstruction[40]: the leaflet is trimmed in length and closed in a tubular shape with a mechanical GIA 75 stapler. This technique is easy and fast; it is precise and allows more regular shape of the graft, facilitating the anastomosis with the vascular stumps.
Reinforced PTFE is the option of choice among synthetic materials; it shows the highest patency rate in the long run when compared to other synthetic conduits, and shortly after the operation, it shows autologous reepithelization.
Postoperative complications of these procedures include problems at the graft anastomosis, graft thrombosis, and infection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree