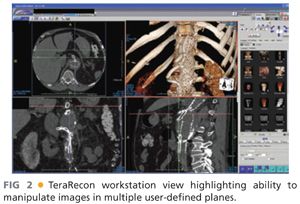
■ Access to a three-dimensional (3-D) workstation/program and familiarity with reconstruction software by the implanting surgeon for manipulation of the images and creating centerline pathways should be mandatory to most accurately plan device orientation, selection, and sizing (FIG 2).
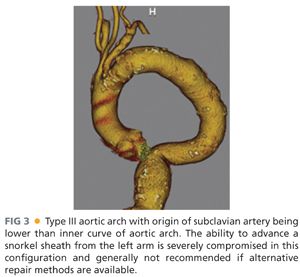
■ Because the snorkel technique usually involves access of the brachial artery for delivery of the parallel visceral stent grafts, visualization of the arch and proximal subclavian is most conveniently obtained by including the chest in the standard CT-A of the abdomen and pelvis. The presence of a challenging type III arch, where the subclavian inserts below the inner curve of the aortic arch, makes the procedure more challenging and many times prohibitive due to concerns about arch manipulation, cerebral emboli, and deliverability of stent grafts (FIG 3). If the patient has already undergone an adequate CT-A abdomen and pelvis and one wishes to avoid the additional contrast load of repeating the study, a noncontrast chest computed tomography (CT) can be performed to visualize the arch but then should be combined with arterial duplex and waveforms of the upper extremities to ensure patency of the axillosubclavian arterial system.
■ For patients with chronic kidney disease, high-grade renal stenosis, atretic kidneys, or multiple visceral and renal vessels involved in the endovascular plan for snorkeling, nuclear medicine split renal function tests can help determine if it is reasonable to sacrifice one of the renal arteries. This can be done in order to simplify the snorkel strategy and keep the number of cranially oriented stent grafts to two, which may have an influence on overall morbidity and mortality from the procedure.1,7,8
SURGICAL MANAGEMENT
Preoperative Planning
■ All patients considered for snorkel/chimney or periscope techniques should have undergone an extensive informed consent discussion related to off-label use of endograft components for treatment of their complex aneurysm. Alternatives often discussed include open surgery with suprarenal clamping, hybrid debranching, referral to a center with access to fenestrated or branched devices, or no surgery at all. Once the decision is made to proceed with the snorkel strategy, we prefer a two-surgeon approach with one performing the femoral access portion and one the brachial access portion. Both surgeons should have reviewed on the 3-D workstation the anatomy, the endovascular plan, and the sequence for deployment.
■ Access to a hybrid endovascular suite is highly recommended, although not mandatory, for successful completion of these procedures. Fixed imaging provides improved accuracy, reliability, and reproducibility of the anatomy throughout the sequence of the snorkel procedure. Knowledgeable operating room and cath-angio staff should be assigned to these cases and available endografts and wires/catheters as well as backups should all be arranged ahead of time to provide the safest working environment for the patient as well as the operative team.
■ Choosing the main body endograft, its configuration, and size has been described by numerous authors who all report excellent results overall with a wide variety of devices and formulas.9 In general, we often “oversized” to about 25% to 30% instead of the typical 15% to 20% for standard EVAR to account for the additional fabric infolding to accommodate the snorkel stent(s).
■ Given the amount of dye often used as well as renal artery manipulation during the most complex of snorkel cases, we prefer to admit the patient the evening before or several hours prior to surgery for additional intravenous hydration when possible.
■ General anesthesia is preferred, with consideration for preoperative lumbar drainage based on risk of spinal cord ischemia. Arterial monitoring, when necessary, is achieved via the right arm. Adequate venous access can consist of either large-bore peripheral intravenous lines (IVs) or a central line. There is usually not a need for autotransfusion or cell saver setups unless an iliac or axillary conduit is planned where there is more potential for early blood loss during the procedure.
Positioning
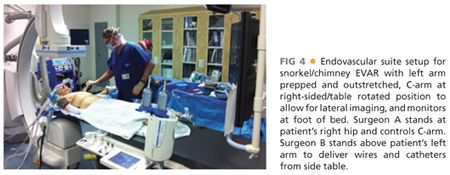
■ The hybrid room can be set up as either “head” position (FIG 4) or “right side, table rotated” depending on the type of imaging equipment. With the right arm tucked, the left arm is prepped circumferentially and placed on an armboard at about 75 to 90 degrees while the chest and abdomen down to the groins are prepped. Surgeon A, who will stand at the patient’s right hip, has control of the C-arm and imaging functions and is in charge of obtaining femoral access and delivery of devices from the groins. Surgeon B stands above the outstretched left arm, with an additional sterile table extending off the left hand to allow for wires and catheters to remain sterile and available for arm access during the procedure. The monitor is placed at a slight angle toward the foot of the bed to allow both surgeons to visualize, or a slave monitor can be employed.
TECHNIQUES
SNORKEL/CHIMNEY ENDOVASCULAR ANEURYSM REPAIR
Arm Access
■ A 5-cm transverse incision slightly below the left axilla over the palpable brachial pulse affords several centimeters of longitudinal exposure of the high brachial artery (FIG 5A). Staying proximal to the deep brachial artery takeoff allows a large enough caliber of brachial artery for typical delivery of two 7-Fr sheaths for a double renal snorkel procedure. At least 7 to 8 cm of healthy brachial artery should be dissected free and slung with vessel loops to allow accurate puncture of the vessel. The two punctures should be placed at least 2 cm apart, and not next to each other, to facilitate later simpler, individual primary closure.
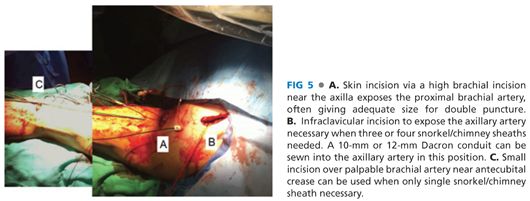
■ For cases when larger delivery sheaths may need to be inserted or in cases where potentially up to three or four snorkel stents need delivery, then an infraclavicular incision and exposure of the axillary artery for possible 10-mm Dacron conduit placement is recommended (FIG 5B). When this is planned, a 20-Fr or 22-Fr sheath can be inserted to get around the arch and then three 6-Fr or 7-Fr sheaths can be used to cannulate the visceral vessels.
■ For the simplest of all snorkel cases, when just one renal artery needs stenting, a lower brachial incision can be made to allow insertion of a single 7-Fr sheath (FIG 5C).
Renal/Visceral Cannulation and Sheath Advancement
■ A 5-Fr micropuncture access is obtained under direct visualization into the brachial artery. A Bentson wire is advanced, under fluoroscopic guidance, most often into the ascending aorta. The use of an Omniflush catheter and glidewire (either a 260-cm Rosen or Amplatz [Cook Medical, Bloomington, IN]) combination, to direct the wire toward the visceral aorta, allows a wire exchange for a stiffer platform. Over this stiffer platform, two 7-Fr 90-cm Pinnacle Destination sheaths (Terumo Medical, Somerset, NJ) are positioned near the visceral target branches to facilitate cannulation attempts (FIG 6A).
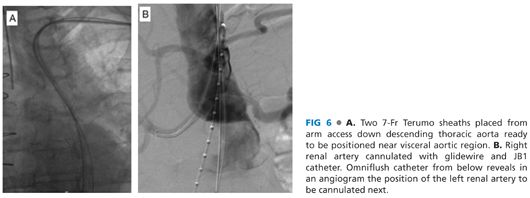
■ Through the 7-Fr sheaths, the targeted renal and visceral branches are cannulated using 260-cm–length hydrophilic guidewires and a 125-cm JB1 catheter (Cook Medical, Bloomington, IN). An angiographic run can be performed from a flush catheter advanced from femoral access to aid in renal cannulation (FIG 6B). Thorough knowledge of the preoperative anatomy, derived from reformatting from the 3-D workstation facilitates this portion of the procedure, guiding optimal angulation of the C-arm.
■ Once cannulated, the sheaths are advanced coaxially into the target artery orifice. When necessary, or in cases where there is a slight turn to the horizontal rather than downward angled, the soft hydrophilic guidewire needs to be exchanged for a 260-cm J-tip Rosen wire (Cook Medical, Bloomington, IN) or Amplatz Superstiff (1-cm tip) to facilitate sheath advancement into the target renal artery. Confirmation angiography, through the sheath, is performed to ensure patency of the renal arteries, cannulation of the main renal artery, and avoidance of accidental side branch cannulation.
Positioning of Main Body Endograft and Snorkel Stent Grafts
■ Standard femoral access for EVAR is employed for snorkel technique. This is well described in other chapters. Briefly, a small transverse incision, below the inguinal ligament, can be used to expose the common femoral artery to the bifurcation for delivery of endograft components. The percutaneous approach involves the “preclose” technique and employs two Perclose ProGlide devices (Abbott Vascular, Santa Clara, CA) oriented at 10 o’clock and 2 o’clock positions.10
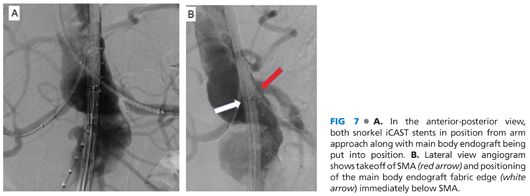
■ The main body endograft can then be delivered up the chosen femoral side to the paravisceral aorta at the same time as the iCAST (Atrium Medical, Hudson, NJ) or Viabahn (Gore Medical, Flagstaff, AZ) stents are advanced through the snorkel sheaths out to the target renal arteries (FIG 7A). The typical length of the iCAST is 59 mm, with the diameter sized appropriately to seal in the target renal artery, most often 5, 6, or 7 mm. For Viabahn stents, similar diameters are used in 50- or 100-mm lengths as appropriate. To prevent theoretical compression of the Viabahn stent by the main body of the endograft, the Viabahn can be reinforced from the inside with a bare-metal, balloon-expandable stent along the areas of overlap with the main body. The positioning of the snorkel stent requires that at least 10 mm of fixation into the renal artery be present and that the proximal extent of the graft is above the fabric of the main body endograft.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


