Open Abdominal Aortic Aneurysm Repair
WILLIAM FORREST JOHNSTON and GILBERT R. UPCHURCH Jr
Presentation
A 65-year-old woman is referred to the office for evaluation of a pulsatile abdominal mass. She states that she first felt the mass 2 years ago, and after telling her primary care physician about it, she was recommended to consult with a vascular surgeon. The mass does not cause her any pain and feels slightly larger to her now than when she initially recognized it. Her past medical history includes hypertension that is well controlled on hydrochlorothiazide and lisinopril and hyperlipidemia controlled with simvastatin. She is a former 40-pack-year smoker that quit 5 years ago. Her past surgical history consists of laparoscopic appendectomy 10 years ago and tonsillectomy when she was a child. On physical examination, she is well appearing with regular heart sounds and bilaterally clear chest sounds. Her abdomen is thin with a nontender pulsatile mass just above the umbilicus. She has 2+ femoral pulses and 1+ dorsalis pedis and posterior tibial pulses with no lower extremity edema.
Differential Diagnosis
Given the pulsatile nature of the abdominal mass, one must consider abdominal arterial pathology. An abdominal aortic aneurysm (AAA) is the most likely cause, although a normal aorta may be palpated in thin patients. Consideration should also be given to malignancy, pancreatic pseudocyst, and liver enlargement from right-sided heart failure. All of the above possibilities can be evaluated with appropriate imaging.
Workup
During the workup of an abdominal mass, an abdominal x-ray is often performed. If the aorta has a calcified wall, an AAA may be apparent on x-ray (Fig. 1A). However, further workup with cross-sectional imaging is needed to confirm an AAA and rule out other causes of pulsatile abdominal masses. Computed tomography (CT) angiography (Fig. 1B) is frequently the preferred imaging modality because it provides high-resolution arterial imaging with contrast delivered through a large-bore peripheral intravenous line. Unlike traditional angiography, no arterial access is required. The most significant limitation of CT angiography is the risk of contrast-induced nephropathy, especially in patients with preexisting renal insufficiency. Contrast-induced nephropathy is the third leading cause of hospital-acquired renal failure. Hydration prior to contrast exposure with bicarbonate solution and administration of N-acetylcysteine may help to prevent renal damage with minimal risk for adverse effects. If the patient has a contrast allergy, she may be pretreated with steroids and diphenhydramine or evaluated with magnetic resonance angiography (MRA). MRA frequently employs gadolinium-based contrast, which is associated with nephrogenic systemic fibrosis (less than 1%) in patients with renal insufficiency. Given the risks of both CT angiography and MRA with gadolinium in patients with renal insufficiency, consideration should be given to imaging patients with baseline renal insufficiency using time-of-flight MRA, diagnostic CO2 angiography, or duplex ultrasound.
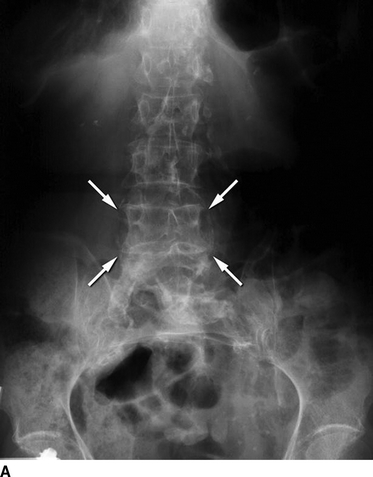

FIGURE 1 A: Abdominal plain film demonstrating a calcified aortic shadow consistent with an AAA (arrows). B: CT angiography demonstrates an AAA with a calcified aortic wall (white arrows) and adjacent mural thrombus.
The maximal aortic diameter should be measured orthogonal to the aortic long axis. This is most easily performed on cross-sectional imaging with fine cuts to allow centerline measurements of aortic diameter, aneurysm length, angularity, extent of calcification, aortic thrombus, and iliac vessel tortuosity. Aortic diameter should be measured perpendicular to the aortic centerline long axis to prevent overestimation of aortic diameter (Fig. 2). In addition to cross-sectional imaging to determine arterial and venous anatomy, the workup of a patient with a suspected AAA should include evaluation of renal and hepatic function, as well as coagulation studies. All patients should receive an EKG to evaluate cardiac function and provide a baseline comparison for postoperative studies. Eagle’s criteria can be applied to determine if further cardiac assessment is indicated. If the patient is not over 70 years old and does not have a history of Q waves on EKG, angina, ventricular ectopy requiring treatment, or diabetes mellitus, he or she is considered low risk for a perioperative cardiac event and no further cardiac evaluation is necessary.


FIGURE 2 Coronal view of the abdominal aorta from CT angiography showing a juxtarenal AAA using (A) centerline measurement compared to (B) multiplanar resolution. Renal artery origin is shown with arrows.
Case Continued
Since the patient has no baseline renal insufficiency, CT angiography is performed and demonstrates an AAA with a maximal aortic diameter of 7.2 cm and a large amount of mural thrombus (Fig. 3). The aneurysm extends proximally and is within 5 mm of the origin of the renal arteries (Fig. 4). No other abdominal pathology is identified on CT. She has normal renal function, electrolytes, liver function tests, hematocrit, and coagulation studies. Since she does not meet any Eagle’s criteria, an EKG is sufficient cardiac evaluation, and she is started on β-blockers to decrease her perioperative cardiac risk.

FIGURE 3 Axial image of CT angiography demonstrates a 7.2-cm AAA with extensive mural thrombus (arrows).

FIGURE 4 Three-dimensional reconstruction of a CT angiogram showing a juxtarenal AAA.
Diagnosis and Treatment
An AAA is defined as enlargement of the aorta to 1.5 times the normal diameter, which is approximately 2 cm (range = 1.9 to 2.3 cm in men, 1.7 to 1.9 cm in women). Therefore, an AAA has been conventionally defined as an aortic diameter greater than 3 cm. AAA repair is intended to prevent aortic rupture, which carries a 75% to 90% risk of mortality. The risk of rupture increases dramatically with increasing aortic size with an aortic diameter of 3 to 4 cm having nearly no risk of rupture, 4 to 5 cm associated with 0.5% to 5% annual rupture risk, 5 to 6 cm associated with 3% to 15% rupture risk, and greater than 8 cm associated with 30% to 50% rupture risk. Since the risk of mortality during open AAA repair is approximately 1% to 5%, elective repair is reserved for an aortic diameter of greater than 5.5 cm in men. Women have smaller aortic diameters at baseline, and a diameter of 5.0 cm may be a more suitable indication for elective repair for women. Most AAAs will progress over time, averaging 0.3 cm/year of expansion. A more rapid expansion rate would prompt earlier consideration for repair, as rapid expansion is associated with an increased risk of rupture. Additional risk factors for aortic rupture include female gender, hypertension, current smoking, chronic obstructive pulmonary disease (COPD), and a family history of AAAs.
If the AAA measures less than 5.5 cm in a man or less than 5.0 cm in a woman, the patient may be conservatively followed with close observation, annual imaging, smoking cessation, and medical treatment of hypertension and hyperlipidemia. While our patient presented with a pulsatile abdominal mass, the majority of AAAs are diagnosed incidentally on diagnostic imaging performed for other reasons. Physical examination alone has a positive predictive value of 15% for diagnosing an AAA greater than 3.5 cm. Regarding AAA screening, population screening with duplex ultrasound decreases AAA-related mortality and is currently an included Medicare benefit for all men over the age of 65 years old who have ever smoked as well as anyone with a family history of AAAs.
Case Continued
The patient is diagnosed with a 7.2-cm juxtarenal AAA. Since her risk of rupture exceeds her risk of operative mortality, she is offered AAA repair. Endovascular and open options are discussed.
Open or Endovascular Approach
Once a patient meets size criteria, he/she should be evaluated for repair with either endovascular aneurysm repair (EVAR) or open repair. EVAR has rapidly become the standard of care for infrarenal aneurysms. Compared to open repair, EVAR is associated with decreased morbidity, operative times, ICU length of stay, and perioperative mortality. Despite the success of EVAR, open repair remains a valuable adjunct to the treatment of patients with AAAs and is indicated in patients with small diameter or severely angulated iliac vessels that cannot accommodate sheath placement, a severely angulated proximal aortic neck, an aortic neck length less than 7 to 15 mm (until fenestrated or multibranch EVARs are approved in the United States), aortoiliac occlusive disease in the distal aorta, a large inferior mesenteric artery (IMA) appearing as the dominant blood supply to the colon, or according to patient preference. Some surgeons also prefer open repair in younger patients under the age of 65 years old since the long-term durability of EVAR remains unknown.
Case Continued
After extensive discussion regarding EVAR compared to open repair, the patient decides to pursue open repair due to the angulation of her aortic neck and the proximity of the renal arteries to the aneurysm.
Surgical Approach
Prior to making an incision, careful review of the preoperative imaging should be performed to determine where aortic clamps will be placed. The proximal clamp may be infrarenal, suprarenal, supramesenteric, or supraceliac depending on the proximal extent of the aneurysm and the amount of calcifications. The duration of aortic occlusion proximal to the renal arteries should be minimized to decrease renal ischemic injury. The infrarenal abdominal aorta may be approached either transabdominal through a midline incision or retroperitoneal from the patient’s left side.
Using a transabdominal approach, a midline laparotomy is performed with reflection of the transverse colon superiorly and retraction of the small bowel to the patient’s right side after division of the ligament of Treitz. Exposure is facilitated with the assistance of a self-retaining retractor system. The posterior peritoneum is incised longitudinally from the iliac arteries to the inferior aspect of the pancreas. Routine preservation of sexual function includes careful retraction of the autonomic nerves coursing on top of the left iliac artery. Additionally, the left ureter may be identified crossing the iliac bifurcation and avoided. The left renal vein is dissected free and may be retracted superiorly to fully identify the aneurysm neck. If needed, the left renal vein may be divided at its junction with the IVC to maintain collateral flow through the left lumbar, gonadal, and adrenal veins.
Once the aorta is dissected, disease-free proximal aorta and iliac arteries should be identified for cross-clamping, avoiding the risk of distal embolization with clamping atherosclerotic plaque. Heparin is given for anticoagulation at a dose of 50 to 100 U/kg. First, vascular clamps are placed across the common iliac arteries. Next, a proximal vascular clamp is placed in the suprarenal position after occlusion of the renal arteries with vessel loops. The aneurysm is incised longitudinally with care taken to avoid the origin of the IMA. Any thrombus or debris is removed from the aortic lumen, and the proximal aorta is fashioned with a horizontal incision just below the renal artery origins for proximal anastomosis. Back bleeding lumbar arteries are encountered posteriorly and ligated with figure-of-eight sutures. The IMA has moderate back bleeding and is oversewn. The proximal anastomosis is performed in end-to-end fashion using polypropylene sutures with large aortic bites and smaller bites on the prosthetic graft. A felt pledget ring may be incorporated into the anastomosis for reinforcement. Once the proximal anastomosis is complete, the proximal clamp is released to check for hemostasis at the suture line. If adequate, the vascular clamp is moved down to the proximal portion of the graft and flow is reestablished to the renal arteries. The proximal anastomosis should be done in the timely fashion, preferable less than 20 to 30 minutes. If distal anastomosis is planned for the terminal aorta, a similar technique is used to attach a graft to the aorta above the bifurcation. Frequently, aneurysm disease will extend to the iliac arteries, requiring placement of a bifurcated graft sutured to disease-free iliac vessels.
After adequate perfusion of the intestines and lower extremities is confirmed, heparin anticoagulation is reversed with protamine sulfate. The anterior arteriotomy is closed over the graft, followed by closure of the retroperitoneum to provide a barrier to the intestines. The small bowel and colon are placed back in their normal position, and the anterior abdominal wall is closed in standard fashion.
If preferred, retroperitoneal exposure may be performed through a midline incision or via a left retroperitoneal incision along the 10th to 12th ribs with the patient rotated onto his right side. The retroperitoneal approach may be associated with decreased postoperative ileus. Dissection is performed in the retroperitoneal plane and may be continued either anterior or posterior to the left kidney. If carried posterior to the kidney, the lumbar vein must be identified and ligated. The ureter is retracted medially with the kidney. Exposure of the right renal artery and right iliac artery can be performed once the aneurysm is entered. Repair is otherwise similar to the transabdominal approach in Table 1.
TABLE 1. Open AAA Repair




