Lung volumes measured during exercise demonstrating development of dynamic hyperinflation. The EELV rises throughout exercise until a critical point is reached and tidal volume can no longer be increased.
History of LVRS
Brantigan[13] was the first to resect emphysematous tissue in an attempt to reduce lung volumes in severe hyperinflated emphysema. While many of the surviving patients reported subjective improvement, the perioperative mortality was 18%, and postoperative lung function was not measured in a systematic manner. The procedure was not widely accepted because of high operative mortality without objective outcomes to document efficacy. The procedure was reintroduced in 1995 after Cooper reported the results of 20 cases of bilateral LVRS demonstrating dramatic improvements in pulmonary physiology and 6-minute walk distances[14]. Shortly thereafter, the same group published their outcomes in 150 consecutive cases of LVRS and reported a 90-day operative mortality of 4%. Postoperative FEV1 (0.70 L [25% predicted] to 1.0 L [38% predicted], p < 0.0001), RV (6.0 L [288% predicted] to 4.3 L [205% predicted], p < 0.001), TLC (8.4 L [143% predicted] to 7.2 L [125% predicted]) all significantly improved when compared to preoperative values[15]. Subsequent to these reports, other uncontrolled series and case reports suggested that LVRS could be beneficial in selected emphysematous patients. The first randomized, controlled trial evaluating the effectiveness of LVRS was published by Criner et al. in 1999[16]. In this study, 37 subjects were randomized to either LVRS or continued pulmonary rehabilitation and optimized medical care, and subjects in the nonsurgical arm could cross over to surgery following the period of continued pulmonary rehabilitation. The authors found that LVRS significantly improved FEV1, TLC, RV, PaCO2 (partial pressure of carbon dioxide), 6-minute walk distance, VO2 (oxygen consumption) on cardiopulmonary exercise testing, and quality of life 3 months following surgery compared to medical therapy[16]. A subsequent randomized, controlled trial comparing LVRS to maximal medical therapy in 48 patients also demonstrated an improvement in FEV1, TLC, RV, and shuttle walk distance[17]. Five of the surviving surgical patients did not have improvement following surgery, and all of these subjects were noted to have diffuse emphysema present on computed tomographic (CT) imaging. Neither of these studies was powered to evaluate survival with LVRS. The Centers for Medicare and Medicaid Services (CMS) noted increased utilization of this procedure code between October 1995 and January 1996; there were 711 LVRS procedures performed with a 1-year mortality of 26%, which was much higher than that being reported in the literature[18]. Both the Agency for Healthcare Research and Quality (AHRQ) and CMS recognized that some patients did benefit from LVRS, but they recommended that a prospective trial be conducted in order to study the effect of LVRS in subjects with comprehensive, long-term postoperative follow-up.
National Emphysema Treatment Trial (NETT) design
The majority of the data regarding LVRS originated from the NETT, a multicenter prospective randomized, controlled trial comparing optimal medical therapy, including pulmonary rehabilitation, to optimal medical therapy plus LVRS[19]. The trial was designed to examine the effect of LVRS on the coprimary endpoints of survival and exercise performance. Secondary outcomes included the effects of LVRS on lung function, patient symptoms, and quality of life compared to maximal medical therapy. NETT sought to enroll patients with moderate to severe bilateral emphysema on high-resolution CT (HRCT) imaging who were free from other diseases that would interfere with either data collection or the subject’s ability to complete the trial. Inclusion and exclusion criteria are listed in Tables 8.1 and 8.2, respectively, but essentially investigators sought to enroll subjects with either heterogeneous or homogeneous disease who were not at high risk for perioperative morbidity and mortality and who were likely to complete the trial[19]. All subjects were optimally medically treated and underwent pulmonary rehabilitation prior to randomization. Pulmonary rehabilitation was conducted in three different phases of the trial including prerandomization (16–20 sessions over 6–10 weeks), postrandomization (10 sessions over 8–9 weeks), and maintenance therapy for study duration.
History and physical exam consistent with emphysema
CT scan evidence of bilateral emphysema
Prerehabilitation postbronchodilator TLC ≥ 100% predicted
Prerehabilitation postbronchodilator RV ≥ 150% predicted
Prerehabilitation FEV1 (maximum of pre- and postbronchodilator values) ≤ 45% of predicted and, if age ≥ 70 years, prerehabilitation, FEV1 (maximum of pre- and postbronchodilator values) ≥15% of predicted
Prerehabilitation room air, resting PaCO2 ≤ 60 mmHg (≤ 55 mmHg in high altitude)
Prerehabilitation room air, resting PaO2 ≥ 45 mmHg (≥ 30 mmHg in high altitude)
Prerehabilitation plasma cotinine ≤ 13.7 ng/ml (if not using nicotine products) or prerehabilitation arterial carboxyhemoglobin ≤ 2.5% (if using nicotine products)
Bodymass index ≤ 31.1 (males) or ≤ 32.3 (females) as of randomization
Nonsmoker (tobacco products) for 4 months prior to initial interview
Approval for surgery by cardiologist if any of the following: unstable angina, left ventricular ejection fraction cannot be estimated from the echocardiogram, left ventricular ejection fraction < 45%, dobutamine-radionuclide cardiac scan indicates coronary artery disease or ventricular dysfunction, > 5 premature ventricular beats/minute (rest), cardiac rhythm other than sinus or premature atrial contractions noted during resting EKG, S3 gallop on physical examination
Completion of all prerehabilitation assessments
Judgment by study physician that patient is likely to be approved for surgery upon completion of the rehabilitation program
Completion of NETT rehabilitation program
Completion of all postrehabilitation and all randomization assessments
CT scan evidence of diffuse emphysema judged unsuitable for LVRS
Previous LVRS (laser or excision)
Pleural or interstitial disease which precludes surgery
Giant bulla (≥ one-third of the volume of the lung)
Clinically significant bronchiectasis
Pulmonary nodule requiring surgery
Previous sternotomy or lobectomy
Myocardial infarction within 6 months of interview and ejection fraction < 45%
Congestive heart failure within 6 months of interview and ejection fraction < 45%
Uncontrolled hypertension (systolic > 200 mmHg or diastolic >110 mmHg)
Pulmonary hypertension: mean PPA on right heart catheterization ≥ 35 mmHg (≥ 38 mmHg in high altitude) or peak systolic PPA on right heart catheterization ≥ 45 mmHg (≥ 50 mmHg in high altitude); right heart catheterization is required to rule out pulmonary hypertension if peak systolic PPA on echocardiogram > 45 mmHg
Unplanned, unexplained weight loss > 10% usual weight in 90 days prior to interview or unplanned, explained weight loss > 10% usual weight in 90 days prior to interview
History of recurrent infections with daily sputum production judged clinically significant
Daily use of > 20 mg of prednisone or its equivalent
History of exercise-related syncope
Resting bradycardia (<50 beats/min), frequent multifocal PVCs (premature ventricular contractions) or complex ventricular arrhythmia or sustained SVT (supraventricular tachycardia)
Cardiac dysrhythmia that poses a risk to the patient during exercise testing or training
Oxygen requirement during resting or oxygen titration exceeding 6 L/min to keep saturation ≥ 90%
Evidence of systemic disease or neoplasia that is expected to compromise survival
Any disease or condition which may interfere with completion of tests, therapy, or follow-up
6 MWD (maximum walking distance) ≤ 140 m postrehabilitation
Inability to complete successfully any of the screening or baseline data collection procedures
Surgical and anesthetic procedures
All centers performed surgical LVRS; 8 of the 17 centers performed median sternotomy (MS), 3 of the 17 centers used video-assisted thorascopic surgery (VATS) approach, and the remaining 6 centers randomized subjects to MS or VATS. Surgeons removed 25 to 30% of the total lung tissue from each lung targeting the most diseased regions, and they were permitted to use buttress suture material to reinforce staples lines at their discretion to minimize postoperative air leaks. All MS subjects received a thoracic epidural catheter for perioperative pain management, and all subjects were expected to be extubated within 2 hours. Additionally, everyone received chest respiratory therapy and physical therapy on the first postoperative day to enhance mobility[19].
Data analysis
The investigators used an intention-to-treat analysis and reported the outcomes of the entire cohort. The NETT steering committee determined a priori that a 10-watt change in exercise performance and an 8-point change in St George’s Respiratory Questionnaire (SGRQ) were clinically meaningful changes for a surgical procedure with significant morbidity and mortality. Because one of the main goals of NETT was to define which subjects would benefit from LVRS a priori, the investigators identified the following prognostic factors prior to initiation of the trial: age, FEV1 percent predicted, PaCO2, RV percent predicted, distribution of perfusion on radionuclide lung scanning, homogeneity or heterogeneity of emphysema distribution on HRCT, and the presence of hyperinflation on chest X-ray[19,20]. During the trial, but well before data collection was completed, the Data and Safety Monitoring Board (DSMB) and steering committee additionally identified the following factors: diffusion capacity of carbon monoxide (DLCO), maximal exercise capacity, RV/TLC ratio, ratio of expired ventilation in 1 minute to carbon dioxide excretion in 1 minute, presence or absence upper lobe predominant emphysema, degree of dyspnea, quality of life, race or ethnic group, and sex.
National Emphysema Treatment Trial (NETT) outcomes
Primary NETT outcomes for all patients
In 2003 the first report of NETT outcomes was published after patients were followed for a mean of 29.2 months[20]. A total of 3,777 subjects were screened and 1,218 were randomized to optimal medical therapy (610 subjects) or LVRS (608) subjects. Nearly all the subjects randomized to LVRS (580/608 [5.4%]) underwent LVRS (406 [70%] by median sternotomy, 174 [30%] by video-assisted thoracoscopic surgery), 21 [3.5%] declined LVRS, and 7 (1.2%) were considered unsuitable by the surgeon for LVRS after randomization. The 90-day mortality rate was significantly higher in the LVRS group (7.9% [95% CI 5.9–10.3] vs. 1.3% [95% CI 0.6–2.6]; p < 0.001) compared to the medical group. During follow-up (mean of 29.2 months) the overall mortality in all patients was not different between LVRS and medical therapy (relative risk [RR] 1.01, p < 0.90) despite an increased early mortality (90-day) in the surgical group[20].
Exercise performance at 6, 12, and 24 months improved by more than 10 watts in 28, 22, and 15% of the subjects undergoing LVRS, respectively, whereas the proportion improving with medical therapy alone was 4, 5, and 3% at the same time points (p < 0.001 for all time points). Patients who underwent LVRS were much more likely to improve FEV1, 6-minute walk distance, dyspnea, and quality of life than those who received medical therapy alone[20].
Identification of the high risk for death with LVRS subgroup
The NETT steering committee predefined a 30-day mortality rate greater than 8% as a stopping endpoint for either group. The investigators found a subgroup of patients who had LVRS with an FEV1 ≤ 20% predicted and either a DLCO ≤ 20% predicted or homogeneous emphysema on HRCT imaging who had a 30-day mortality rate of 16% compared to 0% in the medical group (p < 0.001). Furthermore, those that had survived LVRS had a low likelihood of achieving predefined significant changes in exercise performance and quality of life. Therefore, patients with an FEV1 ≤ 20% predicted who have either a DLCO ≤ 20% or a homogeneous disease should not undergo LVRS due to high morbidity and mortality[21]. After excluding the high-risk group, there were 1,078 remaining subjects in whom the 30-day mortality was 2.2 and 0.2% in the LVRS and medical therapy groups, respectively (p < 0.001). The 90-day mortality rates were also significantly different in LVRS group (5.2%) vs medical therapy (1.5%). The non-high-risk subjects who had LVRS were significantly more likely to have improved 6-minute walk distance, maximal exercise performance, FEV1 percent predicted, and quality of life compared to medical therapy alone[21].
Predicting LVRS outcomes in non-high-risk NETT patients
One of the stated goals of the NETT was to determine which groups of patients with advanced emphysema would benefit from LVRS. NETT demonstrated that the only factors that discriminated mortality differences were the craniocaudal distribution of emphysema on CT imaging and postrehabilitation prerandomization exercise performance differences, while the only factor to discriminate an improvement in maximal exercise performance at 24 months was the craniocaudal distribution of emphysema. Patients were divided into four subgroups based on exercise performance (high vs low) and emphysema distribution (upper lobe predominant vs non–upper lobe predominant). A maximal workload on cardiopulmonary exercise testing was defined as < 25 watts for women and < 40 watts for men. The group with upper lobe predominant emphysema and low exercise performance had a mortality benefit with LVRS compared to medical therapy alone (p = 0.005). Those that had LVRS in this group were more likely to achieve > 10-watt improvement in maximal exercise performance at 24 months (30% vs 0%, p < 0.001) and > 8-point improvement in SGRQ score at 24 months (48% vs 10%, p < 0.001). In the group with upper lobe predominant emphysema and high exercise performance there was no survival advantage with LVRS, although LVRS afforded this group a better chance at a > 10-watt improvement in exercise performance at 24 months (15% vs 3%, p = 0.001) and > 8-point improvement in SGRQ (41% vs 11%, p < 0.001)[20].
Those that underwent LVRS with non–upper lobe predominant emphysema and low baseline exercise performance did not have a survival advantage (p = 0.49) or improved exercise performance at 24 months (12% vs 7%, p = 0.50), but they were more likely to have improved SGRQ at 24 months (37% vs 7%, p = 0.001). Those that underwent LVRS with non–upper lobe predominant emphysema and high baseline exercise performance had increased risk of death (p = 0.02), no difference in exercise performance (3% both groups, p = 1.0), and no difference in SGRQ (15% vs 12%, p = 0.61) at 24 months[20].
In summary, the NETT demonstrated that at a mean follow-up time of 29.2 months, LVRS did not offer a survival advantage compared to optimal medial therapy alone even when the high-risk group was excluded. However, LVRS did result in improved exercise performance, reduction in dyspnea, and improvements in quality of life. Data from NETT suggest that in those with upper lobe predominant emphysema and low baseline exercise performance, LVRS did offer a survival advantage compared to optimal medical therapy alone. The NETT investigators realized that the mean follow-up time of only 2.4 years may not have been long enough and proposed that the group continued to be followed in order to establish the long-term effect of LVRS in this group with advanced emphysema.
Long-term follow-up of NETT subjects
Subjects enrolled in NETT continued follow-up with the clinical centers through June 2004 with scheduled clinic visits, annual testing, and completion of quality-of-life questionnaires. Long-term survival was updated by the clinical centers and through review of the Social Security death file[6]. In all, 1,218 subjects enrolled in NETT with a median follow-up time of 4.3 years, those treated with LVRS demonstrated a long-term survival advantage (Figure 8.2a). The mortality rate was 0.11 deaths per person-year with LVRS and 0.13 medical therapy (RR = 0.85, p = 0.02). Overall, long-term survival improved despite the expected early increase in surgical mortality immediately following LVRS[6]. The survival advantage was similar when the high-risk subjects were excluded from the analysis (RR = 0.82, p = 0.02, Figure 8.2b). Exercise capacity improved by > 10 watts in 23, 15, and 9% following LVRS at 1, 2, and 3 years, respectively, compared to 5, 3, and 1% with medical therapy alone (p < 0.001 at each time point). There was a > 8-point improvement in SGRQ in 40, 32, 20, 10, and 13% following LVRS at 1, 2, 3, 4, and 5 years, respectively, compared to 9, 8, 8, 4, and 7% with medical therapy alone. These differences in SGRQ were significant through 4 years (p < 0.001 years 1 to 3 and p = 0.005 for year 4). In those with upper lobe predominant emphysema and baseline low exercise performance, LVRS markedly decreased mortality (RR = 0.57, p = 0.01, Figure 8.2c) and produced > 10-watt improvement in exercise performance and > 8-point improvement in SGRQ measured quality of life that persisted for 3 years. In the group with upper lobe predominant emphysema and high exercise capacity, there was no survival advantage with LVRS (RR = 0.86, p = 0.19, Figure 8.2d), but more subjects had an increase in exercise performance of > 10 watts and > 8-point improvement in SGRQ during the long-term follow-up period with LVRS[6].
Kaplan-Meier estimates of the cumulative probability of death as a function of years post randomization to LVRS. The overall relative risk (RR) and p value represent the 4.3 years median follow-up. Shown below each plot is the number of subjects at risk in each arm, probability of death in each arm, and the RR (LVRS: Medical) for each year and the p value for difference in the probability.
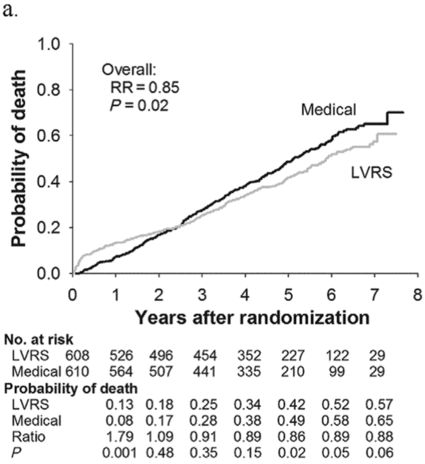
All patients (n = 1,218)
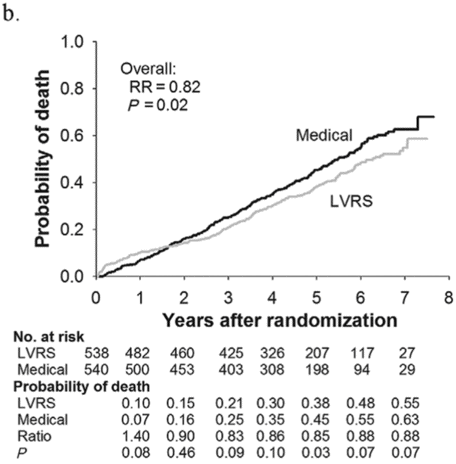
Non-high-risk patients (n = 1,078)
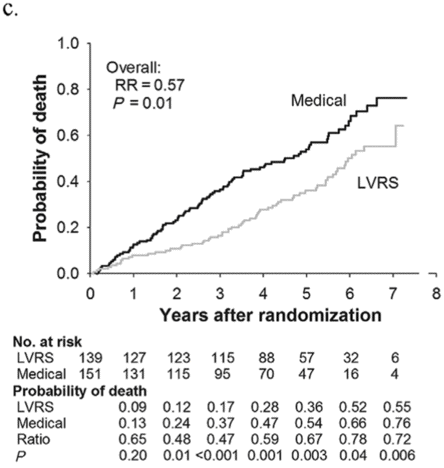
Upper lobe predominant and low baseline exercise performance (n = 290)
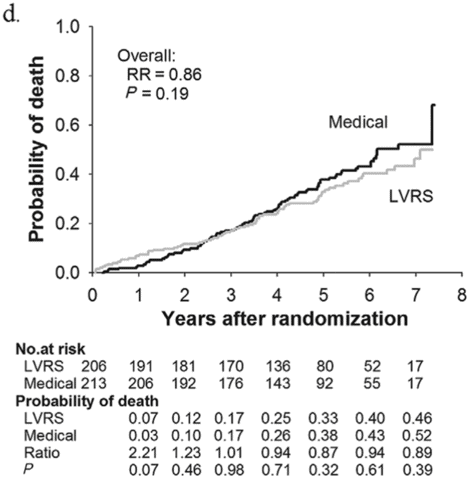
Upper lobe predominant and high exercise capacity (n = 419).
LVRS did not confer a survival benefit or improve exercise performance in the non–upper lobe predo minant emphysema and low exercise group. While subjects who had LVRS were more likely to have had > 8-point improvements in the SGRQ score compared to medical therapy, this advantage dissipated by year 3. Subjects in the non–upper lobe predominant emphysema and high exercise group undergoing LVRS did not have a survival benefit and were not more likely to have significant improvements in either exercise (>10-watt change) or quality of life (>8-point increase in SGRQ) compared to medical therapy alone[6].
Not only did the long-term follow-up of the NETT reaffirm that LVRS is beneficial to subjects with advanced emphysema that have upper lobe predominant emphysema, but it demonstrated that these benefits are durable. Benefits include clinically important improvements in exercise and quality of life, dyspnea, lung function, and in those with baseline low exercise, a survival advantage. Individuals with non–upper lobe dependent emphysema did not have a survival advantage with LVRS (regardless of baseline exercise performance), and although there was improved quality of life in subjects with upper lobe disease and low exercise, it was not durable. Most experts would agree that the risk/benefit ratio in this group is not justified, and those with non–upper lobe predominant disease should not receive LVRS.
In addition to the primary and secondary outcomes that were part of the NETT protocol, there were many other analyses that provided insight into LVRS and the pathophysiology of emphysema.
Operative mortality and cardiopulmonary morbidity following LVRS
Investigators examined data from 511 of the non-high-risk subjects who underwent LVRS and found a 5.5% 90-day mortality rate. The only predictor of operative mortality was the existence of non–upper lobe predominant emphysema with a relative odds (RO) of 2.99 (p = 0.009). During the intraoperative period, 91% of subjects had no complications, 2.2% had transient hypoxemia, and 1.2% developed arrhythmias. Also, 58.7% of LVRS subjects had at least one postoperative complication in the 30-day postoperative time frame, with cardiac arrhythmia being the most common (23.5%). Pneumonia developed in 18.2%, 21.8% required intubation at least once, 11.7% were readmitted to the ICU, and 8.2% required tracheostomy. Only 5.1% of subjects undergoing LVRS were not extubated within 3 days postoperatively[22].
Major pulmonary and cardiovascular 30-day morbidity occurred in 29.8 and 20% of subjects, respectively. Multivariate logistic regression determined that pulmonary morbidity was greater in older patients (RO = 1.05, p = 0.02), lower FEV1 (RO = 0.97, p = 0.05), and lower DLCO (RO = 0.97, p = 0.01). Cardiovascular morbidity was higher with age (RO = 1.07, p = 0.004), preoperative steroid use (RO = 1.72, p = 0.04), and presence of non–upper lobe predominant emphysema (RO = 2.67, p < 0.001)[22].
Air leak following LVRS
Within the 30-day postoperative period, 90% of patients undergoing LVRS had an air leak with a median duration of 7 days, although 12% had an air leak ≥ 30 days. The choice of buttressing technique, stapler brand, or intraoperative adjunctive procedures (tenting or pleurodesis) did not alter either the incidence or duration of air leaks post LVRS. Risk factors for postoperative air leaks were lower DLCO, presence of upper lobe predominant disease, and most important, the presence of pleural adhesions. Air leaks were more prolonged in Caucasians, patients with lower FEV1 or DLCO, presence of upper lobe predominant emphysema, use of inhaled corticosteroids, and presence of pleural adhesions[23]. Subjects having air leaks were more likely to have postoperative complications (57 vs 30%, p = 0.0004) and a longer hospital stay (11.8 ± 6.5 vs 7.6 ± 4.4 days, p = 0.0005). The presence of air leak was not associated with increased mortality and only 4.4% of subjects with an air leak required reoperation.
Median sternotomy (MS) vs video-assisted thorascopic (VATS) approach to LVRS
As mentioned previously, the approach to LVRS was not uniform with MS performed at 8 of the 17 centers, VATS approach used at 3 of the 17 centers and subjects randomized to MS or VATS at the remaining 6 centers. There was no difference in 90-day mortality between the two approaches (5.9% for MS and 4.6% for VATS, p = 0.42)[24]. Intraoperative blood loss and the need for subsequent transfusion of blood products were not different between the two groups either. The mean operating time was 21.7 minutes shorter in the MS group. There were fewer episodes of intraoperative hypoxemia in the MS group (0.8 vs 5.3%, p = 0.004) and overall fewer intraoperative complications in the MS group as well (93.0 vs 86.2%, p = 0.02). There were not any statistically significant differences in postoperative complications between the MS or VATS approaches. Air leak duration was not different between MS and VATS subjects, but median hospital length of stay was shorter with VATS (10 vs 9 days, p = 0.01). At 30 days post LVRS, 70.5% of MS patients and 80.9% of VATS patients were living independently (p = 0.02), but by 4 months post randomization there was no difference in the number of subjects living independently. There were no differences in functional outcomes following LVRS at 12 and 24 months between the two approaches. Total hospital costs at 6 months post LVRS were about $10,000 less with VATS compared to MS ($61,481 ± 3,189 vs $51,053 ± 4,502, p = 0.005)[24]. Overall outcomes, morbidity, and mortality were similar for both MS and VATS, but recovery time and associated costs were lower with VATS.
Effects of LVRS in α-1 antitrypsin-deficient subjects in NETT
There were 16 subjects (1.3%) with severe α-1 antitrypsin (AAT) deficiency (serum AAT level < 80 mg/dL) randomized in NETT, and 10 underwent LVRS. The 2-year mortality was higher with LVRS compared to medical therapy (20 vs 0%) in subjects with AAT deficiency. AAT-deficient subjects also had less improvement in FEV1 and exercise capacity, and the benefits were not as durable[25]. Based on these data and the fact that many AAT-deficient patients have non–upper lobe predominant disease, most centers do not offer LVRS to AAT-deficient patients.
Perfusion scintigraphy and patient selection for LVRS
In NETT 1,045 of the 1,218 subjects had complete perfusion scintigraphy performed at baseline, and a post hoc analysis was performed to determine if perfusion scintigraphy could predict outcome following LVRS[26]. The investigators decided a priori to focus on upper lobe perfusion, and they defined low perfusion to the upper third as being < 20% of total lung perfusion. Among the 248 subjects with upper lobe predominant emphysema and low exercise in NETT, the 202 who had low perfusion to the upper lung zone had decreased mortality with LVRS vs medical therapy (RR = 0.56, p = 0.008) as opposed to the 82 patients with high perfusion to the upper lung zone in whom mortality was unchanged (0.97, p = 0.62). Among the 404 subjects with upper lobe predominant emphysema and high exercise performance, the 278 with low perfusion to the upper lung zone had a reduction in mortality with LVRS compared to medical therapy (RR = 0.70, p = 0.02). In the remaining 126 subjects with upper lobe predominant emphysema and high exercise performance who had high perfusion to the upper lung zone, there was no difference in mortality between LVRS and medical therapy (RR = 1.05, p = 1.0)[26]. In subjects who had non–upper lobe predominant emphysema the perfusion to the upper lung zones did not predict outcomes from LVRS. These data suggest that in those with upper lobe predominant emphysema the presence of low perfusion to the upper lung zone will have improved survival following LVRS. The most likely explanation for this finding is that lung scintigraphy assesses regional lung function in addition to purely anatomic CT imaging.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


