Chapter 116
Diabetic Foot Ulcers
Aksone Nouvong, David G. Armstrong
Based on a chapter in the seventh edition by George Andros and Lawrence A. Lavery
Diabetes is a global epidemic and a leading cause of death by disease. An estimated 366 million people worldwide had diabetes in 2011. This figure is expected to reach 552 million by 2030, corresponding to roughly 8.3% (2011) and 9.9% (2030) of the adult population.1 Diabetes is the most common underlying cause of foot ulcers, infection, and ischemia, which are among the most serious and costly complications of diabetes. Despite advances in the management of diabetes, the rising disease prevalence has resulted in an increased incidence of lower limb amputation due to diabetes.
Epidemiology
As the population ages, the incidence of diabetic foot ulcers (DFUs) and diabetic complications increases. A study of Medicare fee-for-service beneficiaries from 2006 to 2008 reported the incidence of DFUs to be 6.0% and that of lower extremity amputation to be about 0.5%. Among the same population, the prevalence of microvascular and macrovascular complications is approximately 46% and 65%, respectively. The annual mortality rate of patients with DFUs is 11%, and it is 22% in those with a history of lower extremity amputation.2 Patients who undergo a lower extremity amputation have poor quality of life, and the 5-year adjusted mortality rate after a major limb amputation is 46%, which is higher than for many forms of cancer.3
DFUs pose a significant social and economic burden on society. The estimated cost for treatment of one foot ulcer has been calculated at approximately $28,000 during a 2-year period.4 Others have reported that the direct cost estimates (in 2010-adjusted U.S. dollars) range from U.S. $3,096 for a superficial ulcer5 to U.S. $107,900 for an ulcer resulting in amputation.6
Natural History
The natural history of diabetes-related lower extremity amputation can be described as a stairway (Fig. 116-1). The first step is the diagnosis of diabetes, followed by the onset of neuropathy. If an ulcer occurs, it may be complicated by peripheral artery disease (PAD), which slows healing. The coup de grâce is often an ascending infection leading to the urgent need for amputation. There are interventions to prevent each advancing “step” and, ultimately, to prevent a major amputation. It is imperative that the ulcerated diabetic foot be free from infection and receive adequate blood flow to heal in a timely fashion. If one can heal a DFU or at least prevent it from becoming infected, most amputations can be avoided. Interestingly, several reports state that up to 85% of complications, such as amputation, may be preventable.7,8
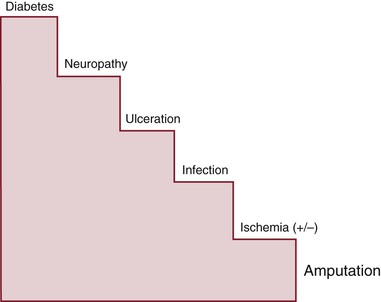
Figure 116-1 Common natural history of major lower extremity amputation. Each step in this “stairway to amputation” is a target for intervention to prevent the escalation to amputation. (From Armstrong DG, et al: Guest editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J 4:286-287, 2007.)
Incidence
Up to 25% of patients with diabetes will suffer from a foot ulcer during their lifetime.3 Ulceration is a pivotal factor in the causal pathway to infection and amputation. Approximately 50% of DFUs become infected, and 20% of these require amputation.3 The incidence of DFU ranges from 2.0% to 6.8% per year in the general diabetes population.6,8–11 Those with diabetes and neuropathy with no other comorbidities will develop an ulcer and represent 7% to 10% of cases annually. Individuals with additional risk factors, such as foot deformity, PAD, previous ulceration, and amputation, will have a 25% to 30% increased risk for ulceration. In more than 85% of lower extremity minor and major amputations, a foot ulcer that subsequently deteriorates to severe infection or gangrene is a critical aspect of the causal pathway.9
It is uncommon for an adult with diabetes to develop a limb infection without a wound as a precipitating factor. Hematogenous soft tissue and bone infections are distinctly unusual. Therefore, it is imperative that foot ulcers be identified and managed promptly.8,12–14 Complications of foot ulcers are the leading cause of hospitalization and amputations. This added burden leads to a 20% to 40% increase of health care resources in diabetes care.9 The most significant cause of amputation after foot ulcers is infection. The presence of PAD increases the risk of this amputation’s being a proximal one.
Recurrence Rate
Reported recurrence rates have been variable but consistently high, ranging from 55% at 12 months to 60% at just 126 days.10,11 In the presence of diabetes, additional comorbidities appear to confer markedly increased risk. For example, the presence of sensory neuropathy or PAD15 increases the risk of these adverse outcomes from 3-fold to 50-fold.14–16
Amputation Rate
Lower extremity amputation may best exemplify the impact of diabetes because it is a measure of end-stage disease and, in many cases, treatment failure. Patients with diabetes are 15 to 30 times more likely to have an amputation than are patients without diabetes2,4,5; 70% to 80% of all nontraumatic amputations occur in people with diabetes.
In the past 15 years, the annual rate of extremity amputation in people with diabetes in the United States has almost halved, to 4.6 per 1000, most of which have been above-the-ankle amputations.12 Although these data are promising, a study from the United Kingdom found that between 1996 and 2005, the number of amputations in people with type 1 diabetes decreased substantially. However, among type 2 diabetic patients, the number of minor amputations almost doubled, and major amputation rates increased more than 40%.12,13 It is estimated that more than 1 million limb amputations are performed on people with diabetes annually, which equates with the loss of limb every 20 seconds somewhere in the world.14
In patients with DFUs, 5% to 8% will require a major amputation within 1 year.15,16 The survival rate in patients with above-the-knee or below-the-knee amputation is 62% at 1 year and 29% at 5 years.17 In patients with peripheral vascular disease or diabetes, progression of their underlying disease can lead to ipsilateral limb reamputation.18–20 Reamputation rates have been noted to be 60% at 5 years. Among patients who underwent forefoot amputation, 79% had reamputation in the first 6 months, 49% had reamputation of the ipsilateral limb within 3 years after the first operation, and 46% died within 3 years.19
Diabetes-related foot complications and amputations are disproportionately more common among men and minorities. These rates are 150% higher in Hispanics and 170% to 240% higher in African Americans in the United States.2 In non-Hispanic whites, 56% of amputations occur in patients with diabetes, whereas 75% of amputations in African Americans and 86% of amputations in Hispanics are due to diabetes.5,8 Across all race and ethnic groups, the incidence of diabetes-related amputations is more than twice as high in men. The risk of amputation in diabetic patients also increases with age.21
Etiology and Pathomechanics
The etiology of DFU is complex and rarely unifactorial. In general, foot ulcers are the cumulative result of repetitive trauma that wears a hole in the skin.6,17,18 The triad of neuropathy, foot deformity, and minor trauma cannot be overemphasized as the major contributing factors of ulcer development. Poor biomechanics causes shear stress and vertical stress to develop on the sole of the foot at the site of high pressures resulting from structural foot deformity and limited joint mobility.13,17,18 Structural deformities, such as claw or hammer toes, first metatarsal joint dislocation, bunion, and limited motion of the ankle and first metatarsophalangeal joint, are often associated with foot ulcers. A combination of clawing of the toes and dislocation of the metatarsophalangeal joints causes retrograde buckling and dislocation. These forces cause the metatarsal head to be pushed in a plantar direction. Ulcers on the great toe often develop because of arthritis or limited motion of the first metatarsophalangeal joint, termed hallux limitus. The presence of neuropathy and hallux limitus has an associated risk ratio of 4.6 for ulceration.22
Ulcers on the tips of clawed toes usually arise because of constant pressure and weight bearing. Ulcers on the metatarsal heads (the “ball” of the foot) occur at sites of high pressure and shear forces that are exposed to repetitive injury during normal walking. Dorsiflexion of the ankle joint should be 10 degrees from neutral, and dorsiflexion of the first metatarsophalangeal joint should be about 50 degrees from neutral. When the ankle motion does not exceed 10 degrees, the abnormality is termed equinus. In diabetic patients, tendons such as the Achilles tendon become glycosylated and less elastic, which can cause equinus leading to increased plantar pressure under the forefoot. A neuropathic patient with decreased ankle joint motion has a risk ratio of 2.3 for development of a forefoot ulcer.22–24 At toe-off in gait, the reduced motion causes more pressure and shear forces under the first metatarsal head (forefoot) or at the hallux interphalangeal joint.23,24
The importance of bone deformities that expose the overlying skin to trauma cannot be overemphasized. Bone prominences in the midfoot often result from Charcot’s neuro-osteoarthropathy, neuropathic fracture, or dysfunction of the tibialis posterior tendon; these areas may ultimately become sites of ulceration. In the presence of sensory neuropathy, a normally painful insult to soft tissues is not recognized until an ulcer develops and is detected by inspection or malodor. Ulcers on the dorsum or sides of the foot are usually due to ill-fitting shoes causing minor trauma. Because patients with diabetic neuropathy lack normal protective sensation, they may select shoes that are too small. Patients may sustain penetrating injuries such as lacerations and puncture wounds that are not recognized owing to the loss of protective sensation.
Risk Assessment
Screening Evaluation
The risk for DFU can be established by a structured screening evaluation. The essential elements of screening include history of foot ulcers, amputations, or lower extremity bypass surgery or angioplasty; inspection of all surfaces of the foot for ulcers or pre-ulcerative lesions; and evaluation for neuropathy, PAD, structural foot deformities, and mobility of the ankle and metatarsophalangeal joints. Screening to identify risk factors in the diabetic foot can be performed by a nurse or trained technician.12,14,17,19 All surfaces of the foot and ankle, including the spaces between all the toes, the soles, and the heels, must be inspected for fissuring, cracks, bullae, calluses, and ulcers. The shoes should also be inspected for sites of wear or pressure and palpated for foreign bodies and irregularities.
Diabetic Neuropathy
Neuropathy affects up to 50% of people with diabetes25,27 and consists of three components: sensory, motor, and autonomic.26,27 Neuropathy itself can also accelerate development of foot deformity,28,29 muscle dysfunction or atrophy, dynamic contracture, and paresis such as footdrop.30 The combined effect of this triad is a foot that cannot respond to pain and is biomechanically impaired, with increased foot pressures, limited joint mobility, and poorly hydrated skin that cannot appropriately respond to injury, predisposing the foot to ulceration. This complication, together with retinopathy, nephropathy, and diabetic arteriopathy, is due to the prolonged effects of hyperglycemia.
Sensory Neuropathy
The damage from sensory neuropathy affects the large myelinated alpha fibers. Its distribution is usually symmetrical in a stocking pattern; as a result, patients are unable to perceive injury to their feet because the primary protective or warning systems are defective. This fundamental pathophysiologic impairment is referred to as loss of protective sensation. Affected patients sustain repetitive, unrecognized injuries to their feet that culminate in full-thickness ulcerations. An ulcer in an insensate foot is usually painless. However, neuropathy can have a wide range of severities and symptoms. Loss of protective sensation does not necessarily mean complete absence of sensation or pain. So-called painful-painless ulcers may develop because of ischemia or deep sepsis; these require prompt attention and intervention. This scenario can also represent damage to both large myelinated nerves and small unmyelinated nerves, so the patient may have burning symptoms because of small-fiber damage and deep, gnawing pain and numbness because of large-fiber neuropathy.
Motor Neuropathy and Resultant Foot Deformity
Motor neuropathy often occurs later in the course of diabetic peripheral neuropathy and contributes to intrinsic muscle wasting of the feet and hands. Motor neuropathy affects the leg and intrinsic foot muscles and changes the biomechanics of the foot, directly contributing to increased shear and pressure under the ball of the foot, the most common site of neuropathic foot ulcers. Severe motor neuropathy contributes to the development of the “intrinsic minus” foot, or the appearance of a high arch structure because of muscle wasting and weakness. Short, weak flexors and extensors that are overpowered by long, stronger flexors and extensors in the foot contribute to structural foot deformities such as hammer or claw toes, dislocated metatarsophalangeal joints, and ankle equinus (Fig. 116-2). Deformities like pes cavus and clawed toes can result in increased pressure at the tips of toes, dorsal aspect of the interphalangeal joints, plantar metatarsal heads, and heel. The increase in pressure during normal ambulation causes callus and ulceration. Neuropathic bone and joint disease (Charcot’s neuro-osteoarthropathy) often affects the midfoot or hindfoot, which can cause severe deformity and plantar ulceration.31


Figure 116-2 Diabetic foot deformity due to motor neuropathy produces pressure points at specific bone prominences (A), which the patient often cannot feel because of sensory neuropathy and loss of protective sensation. Ulceration frequently develops at these sites of increased pressure or shear: hammer toes or claw toes (B), metatarsal head mal perforans ulcer (C), and midfoot collapse or Charcot’s foot (D).
Autonomic Neuropathy
Autonomic neuropathy (sympathetic dysfunction) causes shunting of blood and loss of sweat and oil gland function. The result is dry skin that is prone to cracks and fissures. This is often first manifested as skin breakdown on the heel. The intrinsic “autosympathectomy” caused by autonomic neuropathy explains why surgical sympathectomy fails to improve skin blood flow or to benefit the ulcerated diabetic foot.
Sensory Neuropathy Testing
Even though testing criteria have been established and the tools and tests are inexpensive and noninvasive, neuropathy is often not evaluated. There are several methods to identify sensory neuropathy, including history, vibration perception testing, and pressure assessment. These simple noninvasive investigations have high sensitivity and specificity for the identification of persons with loss of protective sensation and can be performed by nurses or technicians in a few minutes. A simple history of neuropathic symptoms, such as numbness, tingling, burning, or the sensation of insects crawling on the feet (formication), can help identify patients at risk for foot ulcers.32
Patients sometimes mistake the symptoms of diabetic peripheral neuropathy for those of PAD. In addition to reporting numbness, tingling, burning, and formication, they may complain of cold feet despite strong peripheral pulses, an integument that is warm to touch, and no other signs of ischemia. Additional complaints may include a variety of sensations, such as a feeling of thick feet, the sensation that mud is caked on the bottom of the feet, or the feeling of walking in “cement shoes.”
Monofilament Testing
Semmes-Weinstein monofilament testing is one of the most common methods used in the United States to screen for sensory neuropathy.6,19,32 The 10-g monofilament measures pressure sensation and is inexpensive and easy to use. The test apparatus consists of a nylon monofilament attached to a handle; it is designed to provide 10 g of force when it is buckled perpendicular to the test surface of the skin. It is important to explain to the patient that this is not a needle, and a nurse or technician should demonstrate that the monofilament bends on the patient’s hand or arm (Fig. 116-3).

Figure 116-3 A and B, Testing for sensory neuropathy with Semmes-Weinstein monofilament is performed in both feet in 3 to 10 sites, depending on the individual protocol.
The monofilament is pushed perpendicular to the skin with enough pressure to bend the filament, forming a semicircle on the patient’s hand; it is held for approximately 1 second and then removed. Approximately 10 sites on each foot are tested, and the patient is instructed to say yes every time he or she feels pressure or thinks he or she feels pressure. The test is performed with the patient’s eyes closed. Sites to be tested include the first, third, and fifth digits; first, third, and fifth metatarsal heads; base of the fifth metatarsal; heel; arch; and dorsum of the foot.32 Any site at which the patient does not accurately identify the presence of pressure is scored as an abnormal response and is associated with neuropathy with loss of protective sensation.
Vibration Testing
Vibration perception testing, also an alpha myelinated fiber sensory modality, can be evaluated with a 128-Hz tuning fork or a vibration perception threshold testing device. The tuning fork is struck and placed on a bone prominence, such as the great toe or metatarsal head, and the patient is instructed to signify when the vibration stops. The examiner then makes a subjective judgment of whether the level of vibration perception is abnormal. The vibration perception threshold tester is designed to measure vibration sensation on a semiquantitative scale from 0 to 100. The instrument consists of a handpiece with a testing probe on the end, motor, rheostat, and voltmeter. It is applied perpendicular to the distal tip of the erect hallux and is held gently so the weight of the probe is the only applied force (Fig. 116-4). The rheostat is slowly increased until the subject senses the vibration and informs the examiner. Before starting the test, the nurse or technician demonstrates the process on the patient’s hand. The level of perceived vibration is read in volts. Vibration sensation of less than 25 volts has been associated with an increased risk of foot ulceration.33
Peripheral Artery Disease
People with diabetes often have both vascular disease and neuropathy.34,35 PAD has been shown to be present in 20% to 58% of patients with diabetes.16,20,36–39 Therefore, assessment of the vascular supply is crucial.
Anatomy of Peripheral Artery Disease in Patients with Diabetes
Previously, it was erroneously suggested that microangiopathy or small-vessel disease was the primary cause of DFUs. It is now understood that microvascular dysfunction contributes to poor ulcer healing in the neuroischemic diabetic foot36–38 but that macrovascular disease is an important and often treatable contributor.
Microangiopathy is an obstructive arteriolar process that in the past was thought to preclude successful revascularization in diabetic patients.39 Updated studies have now determined that correction of infrapopliteal macrovascular disease often allows wounds to heal but also that microvascular dysfunction is an important component of impaired perfusion in the diabetic foot.39,40 Microvascular dysfunction includes arteriovenous shunting, capillary leakage, precapillary sphincter malfunction, venous pooling, hormonal activity in the vessel, and inflammation in the vessel wall. Impaired perfusion in the diabetic foot is thus complex, and atherosclerosis is not the only cause.39
In patients with diabetes, vascular disease is often localized to the femoropopliteal and tibial segments. The pattern of arterial involvement in diabetes differs from classic atherosclerosis, being characterized by more distal distribution with long segmental occlusions and heavy calcification.33 Although medial calcinosis does not necessarily cause ischemia, it often interferes with indirect measurement of arterial blood pressure.41 In patients with diabetes, collateral formation of large arteries is impaired, causing tissue downstream to be more susceptible to ischemia.42
In many cases, the peroneal artery in the calf remains patent and is the last of the three crural arteries to occlude. It provides pedal circulation through its terminal branches, the anterior and posterior perforating arteries, to collaterals of the dorsal pedal and posterior tibial and plantar arteries. The primary pedal arch is frequently incomplete, but in most cases at least a segment of the plantar arch retains patency if not continuity with the anterior and posterior circulation. Consequently, bypass to a single tibial or peroneal artery usually provides good blood flow to the foot. Infrequently, only a single infrapopliteal arterial segment remains patent, without direct communication to the pedal arteries. In this situation, bypass to the “isolated segment” is the only revascularization option but has a reasonable success rate.23–26 Reports that heel ulcers are slow to heal after bypass to the dorsal pedal artery27 and its collateral runoff suggest that the pedal circulation, somewhat analogous to the coronary circulation, is compartmentalized.23,28 If the ulcer is in the hindfoot, bypass to the posterior tibial–plantar artery axis is preferred. Forefoot and toe ulcers should preferentially receive dorsal pedal bypasses if possible. Midfoot, plantar, and combined toe and heel ulcers can be treated with bifurcated grafts to both the dorsal pedal and posterior tibial branches if these arterial targets are available (Fig. 116-5).

Figure 116-5 If forefoot and hindfoot ulcerations occur in the absence of a patent primary pedal arch, healing may be enhanced by creating a bifurcated bypass to both the anterior and posterior tibial arteries when these are patent, as illustrated in this image. DPA, Dorsal pedal artery; PTA, posterior tibial artery.
It is usually agreed that revascularization is indicated to relieve symptoms of limb-threatening ischemia, including ischemic rest pain, ischemic ulcers, and gangrene. In addition, the International Working Group on the Diabetic Foot (IWGDF) recommends that if severe PAD impairs wound healing, revascularization (endovascular or bypass) should be considered for all ambulatory patients. Exceptions include patients who are severely frail and patients who have short life expectancy (<6-12 months), preexisting severe functional impairment, or a nonsalvageable limb.43
Peripheral Artery Disease Evaluation in the Diabetic Foot
Clinical symptoms are often not helpful in the diagnosis of ischemia in patients with diabetes. DFUs are less often foreshadowed by claudication, a common symptom of PAD in atherosclerotic patients without diabetes. Only 25% of diabetics with PAD report symptoms of intermittent claudication. Either the patient has infrapopliteal arterial occlusions, which do not usually cause claudication, or the patient walks too little or too slowly to experience calf pain. Diabetic patients with ischemia also often lack typical symptoms of claudication or rest pain, probably because such symptoms are masked by underlying peripheral neuropathy.7,33,44,45
In the patient with diabetes, the aortoiliofemoral segment seldom develops occlusive lesions, so buttock and thigh symptoms are rare. The superficial femoral and popliteal arteries are more often affected in patients with diabetes than is the aortoiliac segment, so when claudication is present, it is usually experienced in the calf. Diabetic patients with foot ulcers and gangrene are often found to have a strong popliteal pulse and absent pedal pulses. This finding is due to a highly prevalent pattern of predominantly tibial artery occlusive disease in diabetics; moreover, it portends a high likelihood that the patient is a suitable candidate for revascularization because the peroneal artery or the inframalleolar pedal arteries are usually spared. These general observations underscore the importance of the physical examination in the initial vascular assessment.
The increasing incidence of diabetic vascular disease has led to the emergence of new patterns of diabetic arteriopathy. These lesions include exophytic and “coral reef” plaques and stenoses involving the iliofemoral segment (Fig. 116-6). Arterial calcification, first noted by West46 to be a hallmark of diabetic arteriopathy in the tibial arteries, is now recognized as widespread throughout the arterial system. It frequently results in noncompressible arteries in major arterial segments.

Figure 116-6 Calcific iliofemoral arterial occlusions (arrows) are occurring more frequently and are often associated with “coral reef” exophytic plaques.
The severity of ischemia in the lower limb has a strong predictive value in the outcome of infections,47 wound healing, and level of amputation healing. Optimal diagnostic modalities to accurately predict wound healing and to determine level of amputation are currently not available. Modalities studied to date include ankle-brachial index, segmental blood pressures, arteriography, skin blood flow, skin perfusion pressure, indocyanine green angiography, laser Doppler flowmetry, and transcutaneous oximetry.48,49 None of these tests, either singly or collectively, is completely accurate in the prediction of ulcer healing. Confounding factors such as comorbidities, wound severity, and infection may alter degree of perfusion that is required for healing to occur. A variety of cut points have been used for each of these tests in different study populations, often with different clinical endpoints, making the interpretation of data difficult.
Assessment of arterial perfusion is discussed in more detail elsewhere in this book. Initial evaluation should include inspection for clinical signs such as dependent rubor, pallor with elevation, atrophic integument, and absent hair growth, which are common manifestations of PAD. As people with diabetes and clinically significant PAD are often asymptomatic, guidelines recommend that diabetics have early noninvasive vascular evaluation to identify those who are at risk for poor healing and amputation.
Classification of Risk for Ulceration
Once screening evaluation has been performed for loss of protective sensation, PAD, foot deformity, and history of previous complications, the patient can be placed into an appropriate risk stratum. The classification most widely in use is the American Diabetes Association foot risk system, which involves elements of the IWGDF system as well as subsequent modifications (Table 116-1).14,50 Risk classification assists in the initial triage of the patient and suggests appropriate follow-up intervals, as described later.
Diabetic Foot Ulcers: Assessment, Classification, and Management
Classification of Ulcer Severity
Once one has assessed the extremity for extent of tissue loss (depth), presence of and extent of ischemia, and foot infection, it is often useful to classify wounds to help direct therapy. There are multiple wound classification systems; the Wagner and University of Texas systems are most widely used (Table 116-2).
Meggitt-Wagner System
This system was initially described by Meggitt51 in 1976 and subsequently popularized by Wagner52 in 1981. It uses six wound grades that are mainly based on wound depth and takes into account the presence of osteomyelitis and gangrene. The Wagner system is shown in Table 116-2. This system does not allow the classification of superficial wounds that are infected or wounds of different depths affected by PAD. The Meggitt-Wagner classification system also lacks a clinically relevant and objective measure of PAD.
University of Texas Health Science Center, San Antonio
Another widely used classification system is the University of Texas Health Science Center, San Antonio. This system assesses ulcer depth, presence of wound infection, and presence of lower extremity ischemia. The University of Texas system is based on grades of the wound depth (horizontal axis) and stage of wounds determined by infection and ischemia (vertical axis). The four grades and stages are demonstrated in Table 116-2. Patients with wounds that penetrate to bone with infection and PAD (grade 3D) were 90 times more likely to require amputation than were those with superficial wounds without infection or PAD.53 The rationale for including depth is based on the observation that wounds involving deep structures, such as tendons or joint capsules, are more likely to develop cellulitis, abscess, and osteomyelitis. The rationale for including infection and PAD is that these are two of the factors that most often lead to amputation, poor wound healing, and hospitalization. A comparative study of DFUs by the Wagner and University of Texas classification systems showed a slightly greater association with increased risk of amputation and prediction of ulcer healing with the University of Texas system, which can be used to predict clinical outcome.54,55
Management of Chronic Diabetic Foot Ulcers
The approach to ulcer healing, particularly in the face of a broad list of techniques and technologies, should be systematic. Key elements involve débridement, pressure offloading (whether external by devices or internal by surgery), and wound simplification or closure.
Débridement
Débridement removes devitalized tissue, bioburden, and senescent cells and promotes healing through bleeding. By débridement, a chronic ulcer becomes more of an acute state.56 All necrotic and nonviable tissue should be removed, and there should not be concern about the residual defect caused by the débridement as removal of this tissue is important to attain closure. Most wounds require serial débridement. DFUs that are débrided at each visit have a significantly greater chance of healing in 12 weeks than with débridement less often.57
Several methods of wound débridement exist: mechanical, autolytic, enzymatic, surgical, and biosurgical. Mechanical débridement consists of applying wet gauze dressings, allowing them to dry, and then removing the dry gauze, thereby removing the surface layer (wet-to-dry dressing). This approach has fallen out of favor as a primary method. Autolytic débridement is completed by covering the wound with an occlusive dressing and allowing the ulcer’s proteolytic enzymes to lyse the fibrotic or necrotic tissue. This procedure is not often recommended as there is risk of infection, and more effective methods are available. Enzymatic débridement uses a topical vehicle to remove devitalized tissue. Weak research evidence supports the use of hydrogel. Surgical débridement is the most common and effective method.57 It can be performed with a scalpel, curet, or modalities such as a hydroscalpel or ultrasound with adjustable irrigation systems.58 Hydrotherapy using the Versajet system (Smith & Nephew, Largo, Fla) resulted in shorter débridement time, but no benefit was noted in healing at 12 weeks. Maggot (larval) débridement therapy is considered a biosurgical method.59 There are limited bench-top and cohort studies examining larval therapy as an adjunctive method to reduce the requirement for systemic antimicrobials as well as to improve quality of serial débridement.53,60–62
Dressings
Once an ulcer has been thoroughly débrided, granulation tissue is necessary before wound closure. This process, also called wound bed preparation, starts by achieving an appropriate moisture balance in the wound. The principle of keeping wounds moist without maceration has been demonstrated to accelerate epithelialization.63 On the basis of current research, there is little evidence to support the preference for the use of any one specific dressing or wound application to promote healing of chronic foot ulcers.
Negative Pressure Wound Therapy
Few advances in wound healing have had as significant an impact as negative pressure wound therapy. This technology uses a piece of foam in contact with the wound bed, covered by an occlusive dressing and placed under subatmospheric pressure. The system produces granular tissue that has a characteristic rough appearance. The device can decrease the depth and area of large diabetic foot wounds into a shallow, smaller wound.64 A multicenter randomized controlled trial compared negative pressure wound therapy with advanced moist wound therapy for the treatment of DFUs. The study found reduced time to 90% granulation, wound closure, and amputation with increased incidence of healing by 16 weeks56 (Fig. 116-7).
Platelet and Stem Cell Application
Recent research, including randomized controlled trials, has demonstrated 8- to 12-week full healing of DFUs of 79% to 100% in comparison with control groups ranging from 46% to 62%.65–67 Whereas these data appear promising, much more work is required to confirm or to refute these findings.
Bioengineered Skin and Skin Grafts
After a vascularized wound bed is prepared, wound closure should be performed as rapidly as possible to avoid complications. The IWGDF previously reported improved healing associated with dermal fibroblast culture and fibroblast/keratinocyte co-culture for shallow wounds. Successful healing of DFUs has been seen in up to 51.5% of treated subjects versus 26.3% of standard therapy groups.68 Other types of bioengineered skin have been studied and have shown a higher healing rate and reduction of amputation rate compared with control.60,136
Split-thickness skin grafting is a viable, minimally invasive, and cost-effective method to cover large defects where granulation tissue predominates.58 These grafts perform best on non–weight-bearing surfaces of the foot, including the dorsal, medial, lateral, and arch areas. Although meshed grafting can reduce the rate of seroma and hematoma formation, some have shown that unmeshed grafts perform equally well.62 A retrospective case series reported on the adherence and survival of meshed split-thickness skin grafts with the use of negative pressure wound therapy as a bolster dressing, which reduces seroma and hematoma formation of chronic leg ulcers. The results showed a 93% healing rate of grafts compared with a 67% healing rate in the control group without postoperative negative pressure wound therapy.69 As split-thickness grafts are less suitable for weight-bearing surfaces, some have recommended donor glabrous skin grafts in these locations.70 In a recent review, the authors concluded that split-thickness skin grafts can be used successfully for primary closure of DFUs, despite noting that there is limited research on split-thickness skin grafting.63
In larger soft tissue defects and wounds with exposed deep structures such as bone, more aggressive measures to obtain closure are required. Multiple types of soft tissue flaps can be used to manage these defects. The flaps should be based on current vascular flow and not assumed anatomic flow because patients with diabetes may have segmental or regional arterial occlusions. With fasciocutaneous flaps, the fascia and superficial tissue are rotated into place to cover a defect. One of the more common fasciocutaneous flaps performed in the diabetic foot is a medial plantar artery flap, which can be rotated laterally to cover a subcuboid ulcer or proximally to cover a plantar calcaneal defect. Toe filet flaps can be used to cover submetatarsal head ulcers but require sacrifice of a digit64,71 (Fig. 116-8). Muscle flaps are used to cover exposed bone, such as the extensor digitorum brevis flap and the abductor hallucis or abductor digiti minimi flap. The exposed muscle can be covered with a split-thickness skin graft. Free flaps involve the autotransplantation of a vascularized myocutaneous area to the recipient site and microvascular reanastomosis.72
Nonsurgical Pressure Offloading
Effective pressure reduction is the cornerstone of DFU treatment. Repetitive trauma and pressure on the wound bed are two of the primary reasons for ulcer persistence.73 The accepted “gold standard” treatment of neuropathic diabetic plantar ulcer is the total contact cast. Consistently good outcomes have been demonstrated in many studies.74 However, the device is not widely used because of several concerns, the most common of which is difficulty in application and removal.75 Healing success ranges from 83% to 91% when total contact casting is used.76–78 Compared with removable devices such as cast walkers and half shoes, the total contact cast seems to heal a higher proportion of wounds than the other two modalities do.79
Because increased time and potential cost of weekly cast changes along with a high learning curve may limit the clinical utility of the total contact cast, another option, the instant total contact cast, has been developed. With this method, a removable cast walker is rendered irremovable with the application of a cohesive bandage or cast tape (Fig. 116-9). The instant total contact cast has been shown to have healing rates and total healing similar to those of the total contact cast.78,80-82

Figure 116-9 The instant total contact cast is a removable cast walker rendered irremovable by a cohesive bandage or fiberglass.
In clinical practice, total contact casts and instant total contact casts are better at healing ulcers than removable devices are. This can be explained by the nature of removable devices, for which patient compliance is a factor. One study evaluated the compliance of patients by hiding pedometers inside removable cast walkers and also gave patients pedometers to wear on their hips.80 Subjects were instructed to use the removable cast walkers at all times while ambulating. The investigators found a significant discordance between the hip and removable cast walker pedometers, indicating that the patients were not compliant with instructions to wear their offloading devices at all times and suggesting that improved outcomes would be achieved with a nonremovable device.
Surgical Pressure Offloading
When offloading cannot be accomplished with standard or custom footwear, surgical offloading must be considered. This can be performed with ulcer resection, exostectomy, arthroplasty, reconstruction, or Achilles tendon lengthening. These procedures can be elective, prophylactic, or curative, depending on whether there is absence or presence of neuropathy or open wounds.83
Tendon Lengthening and Transfer.
Several techniques are described in the literature to lengthen the Achilles tendon. The standard Achilles tendon lengthening is accomplished through three stab incisions, posing minimal surgical risk (Fig. 116-10). Although this “lengthening” might be considered more of a “weakening” of the posterior muscle group, it results in muscle tendon balancing and reduces forefoot pressures.84–86 This pressure reduction can lead to ulcer healing and prevent recurrence (Fig. 116-11). In a foot that demonstrates both equinus and varus, in which the foot is inverted and pressure is placed on the plantar or lateral fifth metatarsal area, an additional tendon procedure might be of benefit. The tibialis anterior tendon transfer can be performed through three anterior incisions. The tendon is transected from its normal insertion on the medial cuneiform and transposed laterally into the lateral cuneiform or cuboid; it is usually secured with a bone anchor. This eliminates the forces that pull up on the medial midfoot, transferring pressure laterally. The new function of the tibialis anterior is a more central dorsiflexion action.43

Figure 116-10 Triple hemisection approach to Achilles tendon lengthening. The linear markings connote the outline of the left Achilles tendon medially and laterally. Two transverse stab incisions are made medially and one is made laterally, all 1 cm apart, transecting slightly more than 50% of the tendon substance. The foot is then forcibly dorsiflexed to create a sliding effect of the tendon.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







