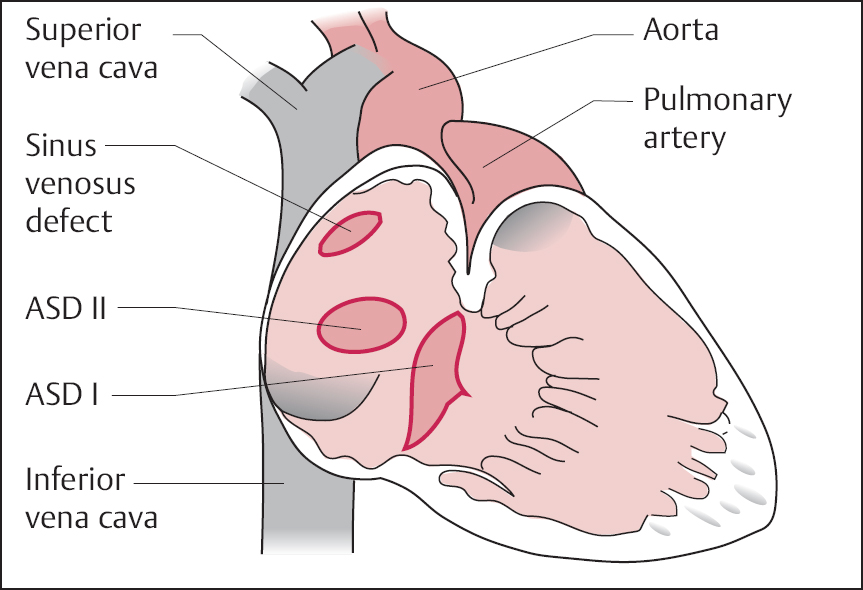
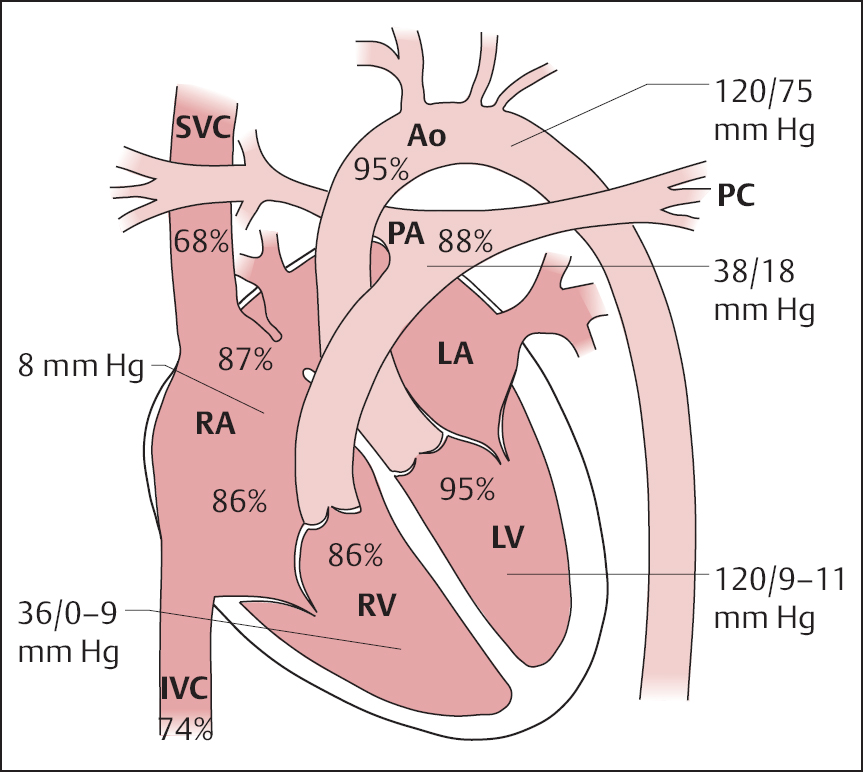
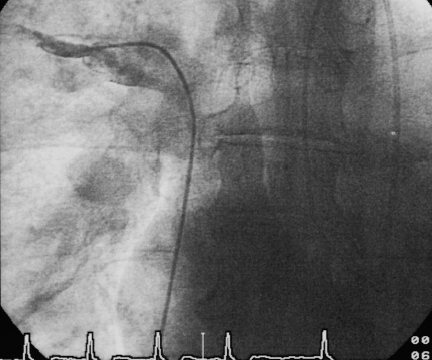
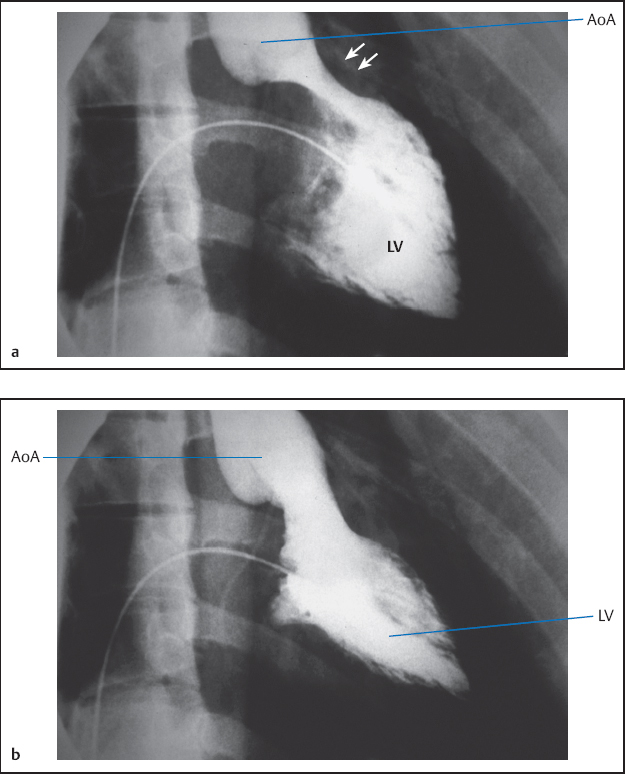
19 Congenital Heart Disease An atrial septal defect (ASD) is present when, secondary to a structural defect, there is a broad connection between the right and left atria. Atrial septal defect is differentiated from persistent foramen ovale, which is not hemodynamically relevant. Atrial septal defects are among the most common congenital heart defects. The female sex is more frequently affected than the male (ratio 2:1). Atrial septal defects can be classified according to their location into sinus venosus defect, ostium secundum defect, and ostium primum defect (Fig. 19.1). The most common is the atrial septal defect of the secundum type (ASD II). The defect is located in the area of the fossa ovalis in the mid- or upper segment of the atrial septum. In some cases a multiperforated septum can be found. In atrial septal defect of the primum type (ASD I) the defect is in the segments close to the base of the atrial septum and directly borders on the atrioventricular valvular plane. Frequently, ASD I is also associated with a cleft formation in the area of the mitral or tricuspid valve. The high atrial septal defect or sinus venosus defect is located between the entry of the superior vena cava and the fossa ovalis, in the posterior cranial part of the atrial septum, so that the superior vena cava rides above the defect. In ~25 % of cases with the ostium secundum defect and in 93 % of cases with the sinus venosus defect, the ASD is combined with one or more aberrant right-sided pulmonary veins (also called “partial anomalous pulmonary venous connection”), predominantly of the right upper pulmonary lobe. With atrial septal defect the shunt volume and the shunt direction are influenced by the size of the defect, the pressure gradient between the left and right atria, and by the distensibility (compliance) of atria and ventricles. The normal pressure difference between the left and right atria is ~4 mm Hg. This difference can also be demonstrated in smaller defects. With larger defects (diameter > 4 cm) there is frequently no pressure gradient between the left and right atria. The left-to-right shunt develops rather as a consequence of the greater distensibility of the right ventricle and right atrium as compared with the left ventricle and left atrium. The shunt causes volume overload of the right heart, as the shunt volume is added to the effective cardiac output, which flows to the right atrium from the caval veins. As a result of this volume overload there is dilatation of the right atrium as well as dilatation and hypertrophy of the right ventricle. Both end-diastolic volume and stroke volume of the right ventricle are therefore larger than in the left ventricle. At the same time there is increased perfusion of the lungs, which with midatrial septal defect can be two to three times the cardiac output of the systemic circulation and in extreme cases can rise five- to six-fold. This increased pulmonary perfusion can lead to a functional pulmonary stenosis with a pressure gradient at the pulmonary valve of more than 20 mm Hg. In ~10 % of patients, the increased pulmonary perfusion results in morphological changes of the pulmonary vessels with a secondary increase in pulmonary vascular resistance. This in turn causes further pressure overload and hypertrophy of the right ventricle with a subsequent decrease in right ventricular compliance and reduction in the shunt volume. Ultimately, a right-to-left shunt can develop (Eisenmenger reaction). If there are aberrant pulmonary veins, the size of the left-to-right shunt is determined by the vessel diameter and/or by the size of the drainage area of the pulmonary veins. Atrial septal defects can usually be diagnosed by auscultation and by echocardiography. The precise anatomy and morphology of the defects can be determined best with transesophageal echocardiography with contrast administration and cardiac MRI; three-dimensional reconstruction of the findings facilitates the planning of an occlusion. If the diagnosis is unambiguous, cardiac catheterization solely to ascertain the diagnosis is therefore not required. However, catheterization is indicated if severe pulmonary hypertension (Eisenmenger reaction) or other associated cardiac or vascular defects are suspected. In addition, in older patients and patients with coronary risk factors, coronary angiography should be performed before planned surgery or intervention. – At rest and – After therapy with inhalation of pure oxygen for 7 to 10 min or (better) – After pharmacological provocation with inhaled iloprost With access via the femoral vein the atrial septal defect of the secundum type can be catheterized relatively easily (e.g., with a multipurpose or Swan-Ganz catheter). The level of the catheter crossing from the right to the left atrium can be used to differentiate between ostium secundum, ostium primum and sinus venosus defect. It also needs to be noted whether an ASD II can be crossed at several locations (evidence for a multiperforated septum). A multipurpose catheter is suitable to engage an aberrant pulmonary vein; with this the superior vena cava and the right atrial wall can be thoroughly and carefully scanned. If necessary the aberrant pulmonary veins can be directly catheterized and also visualized by contrast administration. However, it is also possible that in large atrial septal defects there only appears to be an aberrant pulmonary vein, as in this case the right-sided pulmonary veins run regularly into the left atrium immediately next to the border of the defect and thus can be easily catheterized when searching for a partial anomalous pulmonary venous connection from the right atrium. This can easily lead to misdiagnoses, which can be avoided by pressure recording during catheter pullback from left atrium to right atrium or by angiographic imaging of the pulmonary vein. Reliable imaging of the pulmonary veins is possible in all cases with cardiac CT and cardiac MRI. With a small atrial septal defect the normal characteristics of the atrial pressure tracings are preserved. With larger defects the v-waves of both atrial pressure tracings become more similar to each other due to the equalization of the diastolic pressure. The higher a-wave in the left atrium is mostly preserved. Systolic pressure in the right ventricle is often increased. Across the pulmonary valve there is a moderate, flow-induced pressure gradient of 10 to 30 mm Hg as a sign of the functional pulmonary stenosis due to the shunt volume. Pulmonary vascular resistance is calculated to exclude or classify the severity of pulmonary hypertension. Shunt location and shunt volume are determined by oximetry. Compared with the oxygen saturation in the caval veins, with atrial septal defect the oxygen saturation in the right atrium increases markedly. Oxygen saturations similar to that in the right atrium are, as expected, also found in the right ventricle and in the pulmonary artery. An increase in saturation of > 5 % is considered to indicate a left-to-right shunt. The shunt volume is determined according to the Fick principle. For practical purposes it is usually sufficient to determine the shunt volume as a percentage of the systemic cardiac output. It can be calculated directly from the oxygen saturation values with the following equation: where: % O2PA = oxygen saturation in the pulmonary artery in % % O2art = oxygen saturation in the arterial blood in % % O2ven = oxygen saturation in the mixed-venous blood in % It is worth repeating that the sensitivity of the oxygen method is limited and therefore shunt volumes less than 20 % of the cardiac output are not reliably detected. In contrast, the flat course of the oxygen binding curve at higher levels of oxygen saturation results in inaccurate determinations of larger left-to-right shunts, which can be considerably overestimated. With large left-to-right shunts the oxygen sample from the inferior vena cava should be taken quite caudally, as the pulmonary venous blood can flow across the atrial septal defect into the inferior vena cava as far caudally as the diaphragm. Higher oxygen saturations in the superior vena cava as compared with the inferior vena cava are found in partial anomalous pulmonary venous connection into the superior vena cava. Figure 19.2 shows as an example the pressures and oxygen saturations in a secundum atrial septal defect. The shunt quantification can also reliably be done with cardiac MRI using the four-chamber perfusion measurement by phase-contrast angiography. Angiographic imaging of an atrial septal defect is best done by injection of contrast medium into the area of the entry of the right upper pulmonary vein (pigtail or Berman catheter) in left oblique craniocaudal projection. There is contrast medium flow from the left atrium into the usually enlarged right atrium, with a large left-to-right shunt as far as the inferior vena cava. However, the typically very high cardiac output in the pulmonary circulation leads to a marked dilution of the injected contrast medium, which significantly limits the interpretability of the angiographic images. Due to the good diagnostic capabilities of modern noninvasive imaging modalities (echocardiography and cardiac MRI), direct angiographic imaging of the defect is required only in exceptional cases. In contrast, angiography has more significance in the diagnosis of partial anomalous pulmonary venous connection. Selective injection of contrast into the left and right pulmonary arteries can exclude an anomalous connection. In addition, selective contrast medium injection into a pulmonary vein allows direct imaging of the entry site to demonstrate reliably the correct anatomy or an anomalous connection (Fig. 19.3). A special form of anomalous pulmonary venous return is the scimitar syndrome (a scimitar is a curved sword) where the right pulmonary vein(s) runs into the inferior vena cava combined with hypoplasia of the right lung. Left ventriculography is unremarkable in secundum atrial septal defect. In primum atrical septal defect the atypical position of the mitral valve causes a characteristic atypical form of the left ventricular outflow tract, which is referred to as “goose neck” deformity (Fig. 19.4). Moreover, mitral regurgitation, if present, can be demonstrated and imaged. The decision whether to occlude the defect (interventionally or surgically) or “watch and wait” with regular follow-up depends on the following factors: Table 19.1 provides an overview of the therapy depending on the shunt volume and symptoms. Table 19.1 Therapy of atrial septal defects
Atrial Septal Defect
 Anatomical and Pathophysiological Basics
Anatomical and Pathophysiological Basics
Specific Hemodynamics
 Indication
Indication
 Goals
Goals
 Demonstration of the location of the defect by direct catheterization
Demonstration of the location of the defect by direct catheterization
 Quantitative determination of the shunt volume and shunt direction
Quantitative determination of the shunt volume and shunt direction
 Determination of the pressures in the right ventricle and pulmonary vasculature as well as the pulmonary vascular resistance
Determination of the pressures in the right ventricle and pulmonary vasculature as well as the pulmonary vascular resistance
 Pharmacological provocation (oxygen, nitrates, iloprost, etc.) if required
Pharmacological provocation (oxygen, nitrates, iloprost, etc.) if required
 Evaluate for aberrant pulmonary veins
Evaluate for aberrant pulmonary veins
 Evaluate for mitral regurgitation in mitral valve prolapse or in ostium primum defect
Evaluate for mitral regurgitation in mitral valve prolapse or in ostium primum defect
 Evaluate for additional intracardiac defects
Evaluate for additional intracardiac defects
 Procedure
Procedure
 Venous and arterial puncture
Venous and arterial puncture
 Pressure measurement in the pulmonary and systemic circulation
Pressure measurement in the pulmonary and systemic circulation
 Oximetry run in the pulmonary circulation
Oximetry run in the pulmonary circulation
 Arterial oximetry
Arterial oximetry
 Catheterization of the defect
Catheterization of the defect
 Catheterization of aberrant pulmonary veins (if required with pulmonary angiography)
Catheterization of aberrant pulmonary veins (if required with pulmonary angiography)
 Left ventriculography
Left ventriculography
 Coronary angiography if required
Coronary angiography if required
 Calculation of pulmonary vascular resistance
Calculation of pulmonary vascular resistance
 Findings on Cardiac Catheterization
Findings on Cardiac Catheterization
Catheterization
Pressures
Oximetry
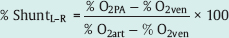
 It is recommended always to do a complete oximetry run with double determination of the saturation values.
It is recommended always to do a complete oximetry run with double determination of the saturation values.
Angiography
 Interpretation of Findings and Patient Management
Interpretation of Findings and Patient Management
 Shunt volume (Qpulm/Qsyst > 1.5 as indication for occlusion)
Shunt volume (Qpulm/Qsyst > 1.5 as indication for occlusion)
 The extent of pulmonary hypertension
The extent of pulmonary hypertension
 The patient’sage
The patient’sage
 The patient’s symptoms
The patient’s symptoms
Hemodynamics | Therapy |
Shunt < 50 % |
|
Asymptomatic patient Symptomatic patient (arrhythmias, pulmonary infections, heart failure) Paradoxical embolisms | Medical therapy (endocarditis prophylaxis with mitral regurgitation) Interventional/surgical therapy Interventional/surgical therapy |
Shunt > 50 % |
|
Asymptomatic and symptomatic patient without pulmonary hypertension | Elective interventional or surgical occlusion |
Shunt > 50 % with pulmonary hypertension |
|
Pulmonary vascular resistance Pulmonary vascular resistance Pulmonary vascular resistance | With reversible pulmonary hypertension: interventional/surgical therapy Potentially lung biopsy: indication for surgery depending on hemodynamic response or morphological changes of the pulmonary vasculature Intervention/surgery contraindicated |
Ventricular Septal Defect
 Anatomical and Pathophysiological Basics
Anatomical and Pathophysiological Basics
Ventricular septal defect (VSD) is the most frequent congenital heart defect in children. In ~25 % of cases the VSD is an isolated defect; in ~30 to 50 % it is combined with other congenital heart defects. VSD also represents ~20 % of congenital defects first diagnosed in adults. Nowadays the initial diagnostic work-up is usually done in infants. In about one-third of cases the defect closes spontaneously during the first years of life.
The most frequent cause of acquired VSD
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree