This chapter shows how the basic principles of cardiovascular physiology that have been discussed apply to the intact cardiovascular system. A variety of normal everyday situations that tend to disturb homeostasis are presented. The key to understanding the cardiovascular adjustments in each situation is to recall that arterial baroreceptor reflex and renal fluid balance mechanisms always act to blunt changes in arterial pressure. The overall result in the healthy individual is that adequate blood flow to the brain and the heart muscle is maintained in any circumstance.
PRIMARY DISTURBANCES AND COMPENSATORY RESPONSES
![]() The cardiovascular alterations in each of the following examples are produced by the combined effects of (1) the direct influences of the primary disturbance on the cardiovascular variables and (2) the reflex compensatory responses that are triggered by these primary disturbances. The general pattern of reflex responses is similar in all situations. Rather than trying to memorize the cardiovascular alterations that accompany each situation, the student should strive to understand each response in terms of the primary disturbances and reflex compensatory responses involved. To aid in this process, a list of key cardiovascular variables and their determinants may be found in Appendix C. If the student understands all the relationships indicated in Appendix C, they will have mastered the core of cardiovascular physiology.
The cardiovascular alterations in each of the following examples are produced by the combined effects of (1) the direct influences of the primary disturbance on the cardiovascular variables and (2) the reflex compensatory responses that are triggered by these primary disturbances. The general pattern of reflex responses is similar in all situations. Rather than trying to memorize the cardiovascular alterations that accompany each situation, the student should strive to understand each response in terms of the primary disturbances and reflex compensatory responses involved. To aid in this process, a list of key cardiovascular variables and their determinants may be found in Appendix C. If the student understands all the relationships indicated in Appendix C, they will have mastered the core of cardiovascular physiology.
A list of important study questions is supplied for Chapters 10 and 11. These questions are intended to reinforce the student’s understanding of complex cardiovascular responses and provide a review of basic cardiovascular principles.
EFFECT OF RESPIRATORY ACTIVITY
![]() The physical processes associated with inhaling air into and exhaling air out of the lungs can have major effects on venous return and cardiac output. During a normal inspiration, intrathoracic pressure falls from approximately negative 2 mm Hg to approximately negative 7 mm Hg (compared to atmospheric pressure) as the diaphragm contracts and the chest wall expands. It rises again by an equal amount during expiration. These periodic pressure fluctuations not only promote air movement into and out of the lungs but also are transmitted through the thin walls of the great veins in the thorax to influence venous return to the heart from the periphery. Because of the unidirectional nature of venous valves, venous return is increased more by inspiration than it is decreased by expiration. The net effect is that venous return from the periphery is generally facilitated by the periodic fluctuations in central venous pressure caused by respiration. This phenomenon is often referred to as the “respiratory pump.”
The physical processes associated with inhaling air into and exhaling air out of the lungs can have major effects on venous return and cardiac output. During a normal inspiration, intrathoracic pressure falls from approximately negative 2 mm Hg to approximately negative 7 mm Hg (compared to atmospheric pressure) as the diaphragm contracts and the chest wall expands. It rises again by an equal amount during expiration. These periodic pressure fluctuations not only promote air movement into and out of the lungs but also are transmitted through the thin walls of the great veins in the thorax to influence venous return to the heart from the periphery. Because of the unidirectional nature of venous valves, venous return is increased more by inspiration than it is decreased by expiration. The net effect is that venous return from the periphery is generally facilitated by the periodic fluctuations in central venous pressure caused by respiration. This phenomenon is often referred to as the “respiratory pump.”
Because of these cyclic changes in intrathoracic pressure, normal breathing is associated with transient changes in heart rate, cardiac output, and arterial pressure. Heart rate in healthy individuals usually fluctuates in synchrony with the respiratory rate. This is referred to as “normal sinus arrhythmia.” There are several factors that contribute to this normal arrhythmia.
1. Cyclical alterations in intrathoracic pressure with normal breathing evoke primary disturbances in blood flow and distribution within the cardiovascular system. Some of these disturbances and compensatory responses are illustrated in Figure 10–1. Filling of the right side of the heart is transiently increased during inspiration and, by Starling’s law, stroke volume and thus cardiac output are transiently increased. In addition, the reduction in pulmonary vascular resistance that accompanies inspiration reduces the right ventricular afterload, which contributes to a transient increase in right ventricular stroke volume. Because changes in output of the right side of the heart induce changes in output of the left side of the heart within a few beats, the net effect of inspiration will be a transient increase in stroke volume and cardiac output from the left ventricle. This will lead to a transient increase in arterial pressure and a transient increase in firing of the arterial baroreceptors. The output of these high-pressure arterial baroreceptors will act on the medullary cardiovascular centers to produce reflex adjustments to lower arterial pressure, by increasing cardiac parasympathetic nerve activity, decreasing sympathetic nerve activity, and causing a decrease in heart rate.

Figure 10–1. Cardiovascular effects of respiratory inspiration.
2. The inspiration-induced decrease in intrathoracic pressure will also stretch low-pressure cardiopulmonary baroreceptors in the vascular and cardiac walls and will increase their firing rate. These low-pressure baroreceptor inputs will add to the information from the high-pressure baroreceptors and promote similar pressure-lowering outputs from the medullary cardiovascular centers.
3. Lung mechanoreceptors located primarily within the airways are also stretched during normal inspiration. Unlike the first two mechanisms, their input into the medullary centers results in an inhibition of the normal tonic vagal activity to the sinoatrial node, causing a transient increase in the heart rate.
Under normal resting conditions, the cyclic change in heart rate is the most apparent cardiovascular response to respiration. However, because of the complicated and sometimes conflicting mechanisms involved in altering vagal tone to the SA node, specific phase relationships between the respiratory cycle and the cardiovascular effects are hard to predict and are greatly influenced by respiratory rate and depth as well as the current average heart rate. However, absence of a respiratory arrhythmia is clearly abnormal.
There are a number of instances when cardiovascular effects of respiratory efforts are exaggerated and extremely important. The following lists several of these situations:
1. During exercise, a deep and rapid breathing rate contributes significantly to the venous return by exaggerating the fluctuations in intrathoracic pressure. This is an important example of the respiratory pump.
2. Yawning is a complex event that includes a significant transient decrease in intrathoracic pressure that is highly effective in increasing venous return (especially when combined with stretching).
3. In contrast to yawning, coughing is associated with an increase in intrathoracic pressure and, if occurring as a prolonged “fit,” can lead to compression of the thoracic vessels, reduced venous return, and such severe reductions in cardiac output as to cause fainting.
4. The Valsalva maneuver is a forced expiration against a closed glottis commonly performed by individuals during defecation (“straining at stool”), or when attempting to lift a heavy object. There are several phases in this cardiovascular reaction. At the initiation of the Valsalva maneuver, arterial pressure is abruptly elevated for several beats due to the intrathoracic pressure transmitted to the thoracic aorta. Sustained elevation in intrathoracic pressure then leads to a fall in venous return and a fall in blood pressure, which evokes a compensatory reflex increase in the heart rate and peripheral vasoconstriction. (During this period, the red face and distended peripheral veins are indicative of high peripheral venous pressures.) At the cessation of the maneuver, there is an abrupt fall in pressure for a couple of beats due to the reduction of intrathoracic pressure. Venous blood then moves rapidly into the central venous pool; stroke volume, cardiac output, and arterial pressure increase rapidly; and a reflex bradycardia occurs. The combination of an episode of high peripheral venous pressure followed by an episode of high arterial pressure and pulse pressure is particularly dangerous for people who are candidates for cerebral vascular accidents (strokes) because this combination may rupture a vessel.
5. Artificial support of respiration with positive-pressure ventilators is sometimes necessary for assuring proper gas exchange in the lungs but does have significant adverse cardiovascular consequences. When the lungs are inflated artificially by such ventilators, intrathoracic pressure goes up (rather than down, as occurs during normal inspiration). Thus, instead of the normal respiratory pump increasing venous return during inspiration, the positive-pressure ventilator decreases venous return during lung inflation. In addition, the increase in intrathoracic pressure tends to compress the pulmonary microcirculation and this increases right ventricular afterload. Therefore, when considering the option of putting someone on a respirator, the benefits of improving pulmonary ventilation need to be weighed against the negative effects on the cardiovascular system.
EFFECT OF GRAVITY
Responses to Changes in Body Position
![]() Significant cardiovascular readjustments accompany changes in body position because gravity has an effect on pressures within the cardiovascular system. In the preceding chapters, the influence of gravity was ignored and pressure differences between various points in the systemic circulation were related only to flow and vascular resistance (ΔP =
Significant cardiovascular readjustments accompany changes in body position because gravity has an effect on pressures within the cardiovascular system. In the preceding chapters, the influence of gravity was ignored and pressure differences between various points in the systemic circulation were related only to flow and vascular resistance (ΔP = ![]() × R). As shown in Figure 10–2, this is approximately true only for a recumbent individual. In a standing individual, additional cardiovascular pressure differences exist between the heart and regions of the body that are not at the heart level. This is most important in the lower legs and feet of a standing individual. As indicated in Figure 10–2B, all intravascular pressures in the feet may be increased by 90 mm Hg simply from the weight of the blood in the arteries and veins leading to and from the feet. Note by comparing Figure 10–2A and B that standing upright does not in itself change the flow through the lower extremities, because gravity has the same effect on arterial and venous pressures and thus does not change the arteriovenous pressure difference at any one height level. There are, however, two major direct effects of the increased pressure in the lower extremities, which are shown in Figure 10–2B: (1) the increase in venous transmural pressure distends the compliant peripheral veins in the legs and feet and greatly increases the blood in these veins by as much as 500 mL in a normal adult and (2) the increase in capillary transmural hydrostatic pressure causes a tremendously high transcapillary filtration rate in the lower legs and feet.
× R). As shown in Figure 10–2, this is approximately true only for a recumbent individual. In a standing individual, additional cardiovascular pressure differences exist between the heart and regions of the body that are not at the heart level. This is most important in the lower legs and feet of a standing individual. As indicated in Figure 10–2B, all intravascular pressures in the feet may be increased by 90 mm Hg simply from the weight of the blood in the arteries and veins leading to and from the feet. Note by comparing Figure 10–2A and B that standing upright does not in itself change the flow through the lower extremities, because gravity has the same effect on arterial and venous pressures and thus does not change the arteriovenous pressure difference at any one height level. There are, however, two major direct effects of the increased pressure in the lower extremities, which are shown in Figure 10–2B: (1) the increase in venous transmural pressure distends the compliant peripheral veins in the legs and feet and greatly increases the blood in these veins by as much as 500 mL in a normal adult and (2) the increase in capillary transmural hydrostatic pressure causes a tremendously high transcapillary filtration rate in the lower legs and feet.

Figure 10–2. The effect of gravity on pressures within a simulated peripheral vascular system. (A) System in a recumbent position; (B–E) system in an upright position; (B) with no compensatory influences; (C) with sympathetic stimulation; (D and E) with the effect of the skeletal muscle pump.
For reasons to be described, a baroreceptor-induced reflex activation of sympathetic nerves accompanies the transition from a recumbent to an upright position. However, Figure 10–2C shows how vasoconstriction from sympathetic activation is only marginally effective in ameliorating the adverse effects of gravity on the lower extremities. Arteriolar constriction can cause a greater pressure drop across arterioles, but this has only a limited effect on capillary pressure because venous pressure remains extremely high. Filtration will continue at a very high rate. In fact, the normal cardiovascular reflex mechanisms are alone incapable of dealing with upright posture without the aid of the “skeletal muscle pump.” A person who remained upright without intermittent contraction of the skeletal muscles in the legs would lose consciousness in 10 to 20 min because of the decreased brain blood flow that would stem from sequential steps of diminished central blood volume, leading to reduced stroke volume, depressed cardiac output, and finally lowered arterial pressure.
Effectiveness of the skeletal muscle pump in counteracting venous blood pooling and edema formation in the lower extremities during standing is illustrated in Figure 10–2D and E. Compression of vessels during skeletal muscle contraction expels both venous blood and lymphatic fluid from the lower extremities (Figure 10–2D). Immediately after a skeletal muscle contraction, both veins and lymphatic vessels are relatively empty because their one-way valves prevent the backflow of previously expelled fluid (Figure 10–2E). Most importantly, the weight of the venous and lymphatic fluid columns is temporarily supported by the closed one-way valve leaflets. Consequently, venous pressure is drastically lowered immediately after skeletal muscle contraction and rises only gradually as veins refill with blood from capillaries. Thus, capillary pressure and transcapillary fluid filtration rate are dramatically reduced for some period after a skeletal muscle contraction. Periodic skeletal muscle contractions can keep the average value of venous pressure at levels that are only moderately above normal. This, in combination with an increased pressure drop across vasoconstricted arterioles, prevents capillary pressures from rising to intolerable levels in the lower extremities. Some transcapillary fluid filtration is still present, but the increased lymphatic flow resulting from the skeletal muscle pump is normally sufficient to prevent noticeable edema formation in the feet.
![]() Actions of the skeletal muscle pump, however beneficial, do not completely prevent a rise in the average venous pressure and blood pooling in the lower extremities on standing. Therefore, assuming an upright position upsets the cardiovascular system and elicits reflex cardiovascular adjustments, as shown in Figure 10–3.
Actions of the skeletal muscle pump, however beneficial, do not completely prevent a rise in the average venous pressure and blood pooling in the lower extremities on standing. Therefore, assuming an upright position upsets the cardiovascular system and elicits reflex cardiovascular adjustments, as shown in Figure 10–3.
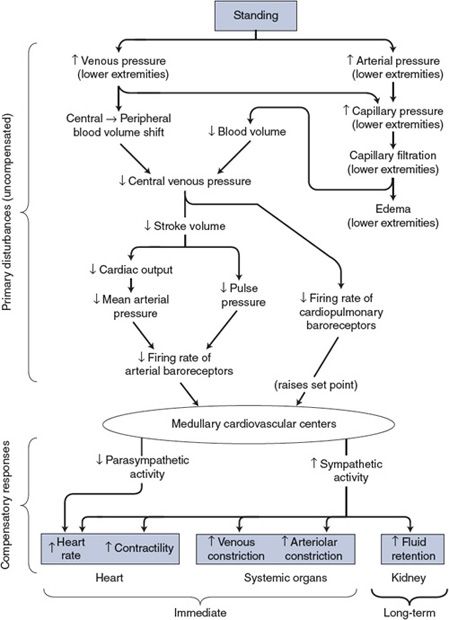
Figure 10–3. Cardiovascular mechanisms involved when changing from a recumbent to a standing position.
As with all cardiovascular responses, the key to understanding the alterations associated with standing is to distinguish the primary disturbances from the compensatory responses. As shown in the top part of Figure 10–3, the immediate consequence of standing is an increase in both arterial and venous pressures in the lower extremities. The latter causes a major redistribution of blood volume out of the central venous pool. By the chain of events shown, the primary disturbances influence the cardiovascular centers by lessening the normal input from both the arterial and the cardiopulmonary baroreceptors.
The consequence of a decreased baroreceptor input to the cardiovascular centers will be reflex adjustments (ie, the compensatory response) appropriate to increase blood pressure—that is, decreased cardiac parasympathetic nerve activity and increased activity of the cardiovascular sympathetic nerves, as shown in the bottom part of Figure 10–3. The heart rate and cardiac contractility will increase, as will arteriolar and venous constriction in most systemic organs (the brain and the heart excepted).
Heart rate and total peripheral resistance are higher when an individual is standing than when lying down. Note that these particular cardiovascular variables are not directly influenced by standing but are changed by the compensatory responses. Stroke volume and cardiac output, conversely, are usually decreased below their recumbent values during quiet standing despite the reflex adjustments that tend to increase them. This is because the reflex adjustments do not quite overcome the primary disturbance on these variables caused by standing. This is in keeping with the general dictum that short-term cardiovascular compensations never completely correct the initial disturbance.
Mean arterial pressure is often found to increase when a person changes from the recumbent to the standing position. At first glance, this is a violation of many rules of cardiovascular system operation. How can compensation be more than complete? Moreover, how is increased sympathetic activity compatible with higher-than-normal mean arterial pressure in the first place? In the case of standing, there are many answers to these apparent puzzles. First, the average arterial baroreceptor discharge rate can actually decrease in spite of a small increase in mean arterial pressure if there is simultaneously a sufficiently large decrease in pulse pressure. Second, the influence on the medullary cardiovascular centers from cardiopulmonary receptors is interpreted as a decrease in blood volume and may raise arterial pressure by mechanisms raising the set point. Third, mean arterial pressure determined by sphygmomanometry from the arm of a standing individual overestimates the mean arterial pressure actually being sensed by the baroreceptors in the carotid sinus region of the neck because of gravitational effects.
The kidney is especially susceptible to changes in sympathetic nerve activity (as discussed in the previous chapter and shown in Figure 9–6). Consequently, as shown in Figure 10–3, every reflex alteration in sympathetic activity has influences on fluid balance that become important in the long term. Standing, which is associated with an increase in sympathetic tone, ultimately results in an increase in fluid volume. The ultimate benefit of this is that an increase in blood volume generally reduces the magnitude of the reflex alterations required to tolerate upright posture.
Responses to Long-Term Bed Rest (or to Zero Gravity)
The cardiovascular system of an individual who is subjected to long-term bed rest undergoes a variety of adaptive changes that are quite similar to those experienced by people who travel outside the earth’s atmosphere at zero gravity. In both cases, the consequences of these adjustments are substantial.
![]() The most significant immediate change that occurs on assuming a recumbent position (or entering a gravity-free environment) is a shift of fluid from the lower extremities to the upper portions of the body. The consequences of this shift include distention of the head and neck veins, facial edema, nasal stuffiness, and decreases in calf girth and leg volume. In addition, the increase in central blood volume stimulates the cardiopulmonary mechanoreceptors, which influence renal function by neural and hormonal pathways to reduce sympathetic drive and promote fluid loss. The individual begins to lose weight and, within a few days, becomes hypovolemic (by normal earth standards).
The most significant immediate change that occurs on assuming a recumbent position (or entering a gravity-free environment) is a shift of fluid from the lower extremities to the upper portions of the body. The consequences of this shift include distention of the head and neck veins, facial edema, nasal stuffiness, and decreases in calf girth and leg volume. In addition, the increase in central blood volume stimulates the cardiopulmonary mechanoreceptors, which influence renal function by neural and hormonal pathways to reduce sympathetic drive and promote fluid loss. The individual begins to lose weight and, within a few days, becomes hypovolemic (by normal earth standards).
When the bedridden patient initially tries to stand up (or when the space traveler reenters the earth’s gravitational field), the normal responses to gravity, as described in Figure 10–3, are not as effective, primarily because of the substantial decrease in circulating blood volume. Upon standing, blood shifts out of the central venous pool into the peripheral veins, stroke volume falls, and the individual often becomes dizzy and may faint because of a dramatic fall in blood pressure. This phenomenon is referred to as orthostatic or postural hypotension. Because there are other cardiovascular changes that may accompany bed rest (or space travel), complete reversal of this orthostatic intolerance may take several days or even weeks.
Efforts made to diminish the cardiovascular changes for the bedridden patient may include intermittent sitting up or tilting the bed to lower the legs and trigger fluid retention mechanisms. Efforts made in space to accomplish the same end may include exercise programs, lower-body negative-pressure devices, and salt and water loading. (To date, these interventions have met with limited success.)
EFFECT OF EXERCISE
Responses to Acute Exercise
Physical exercise is one of the most ordinary, yet taxing, situations with which the cardiovascular system must cope. The specific alterations in cardiovascular function that occur during exercise depend on several factors including (1) the type of exercise—that is, whether it is predominantly “dynamic” (rhythmic or isotonic) or “static” (isometric), (2) the intensity and duration of the exercise, (3) the age of the individual, and (4) the level of “fitness” of the individual. The example shown in Figure 10–4 is typical of cardiovascular alterations that might occur in a normal, untrained, middle-aged adult doing a dynamic-type exercise such as running or dancing. Note especially that heart rate and cardiac output increase greatly during exercise and that mean arterial pressure and pulse pressure also increase significantly. These alterations ensure that increased metabolic demands of the exercising skeletal muscle are met by appropriate increases in skeletal muscle blood flow. (Use the data in this figure to answer Study Questions 10–5 to 10–8.)

Figure 10–4. Changes in cardiovascular variables with strenuous exercise.
![]() Many of the adjustments to exercise are due to a large increase in sympathetic activity, which results from the mechanisms outlined in Figure 10–5. One of the primary disturbances associated with the stress and/or anticipation of exercise originates within the cerebral cortex and exerts an influence on the medullary cardiovascular centers through corticohypothalamic pathways. This set point–raising influence, referred to as the “central command,” causes mean arterial pressure to be regulated to a higher-than-normal level (see Appendix E, Figure E–1). Also indicated in Figure 10–5 is the possibility that a second set point–raising influence may reach the cardiovascular centers from chemoreceptors and mechanoreceptors in the active skeletal muscles. Such inputs would also contribute to the elevations in sympathetic activity and mean arterial pressure that accompany exercise.
Many of the adjustments to exercise are due to a large increase in sympathetic activity, which results from the mechanisms outlined in Figure 10–5. One of the primary disturbances associated with the stress and/or anticipation of exercise originates within the cerebral cortex and exerts an influence on the medullary cardiovascular centers through corticohypothalamic pathways. This set point–raising influence, referred to as the “central command,” causes mean arterial pressure to be regulated to a higher-than-normal level (see Appendix E, Figure E–1). Also indicated in Figure 10–5 is the possibility that a second set point–raising influence may reach the cardiovascular centers from chemoreceptors and mechanoreceptors in the active skeletal muscles. Such inputs would also contribute to the elevations in sympathetic activity and mean arterial pressure that accompany exercise.

Figure 10–5. Cardiovascular mechanisms involved during exercise.
![]() A major primary disturbance on the cardiovascular system during dynamic exercise, however, is the great decrease in total peripheral resistance caused by metabolic vasodilator accumulation and decreased vascular resistance in the active skeletal muscle. As indicated in Figure 10–5, decreased total peripheral resistance is a pressure-lowering disturbance that elicits a strong increase in sympathetic activity through the arterial baroreceptor reflex.
A major primary disturbance on the cardiovascular system during dynamic exercise, however, is the great decrease in total peripheral resistance caused by metabolic vasodilator accumulation and decreased vascular resistance in the active skeletal muscle. As indicated in Figure 10–5, decreased total peripheral resistance is a pressure-lowering disturbance that elicits a strong increase in sympathetic activity through the arterial baroreceptor reflex.
Although mean arterial pressure is above normal during exercise, the decreased total peripheral resistance causes it to fall below the elevated level to which it would be regulated by the set point–raising influences on the cardiovascular center alone. The arterial baroreceptor reflex pathway responds to this circumstance with a large increase in sympathetic activity. Thus, the arterial baroreceptor reflex is responsible for a large portion of the increase in sympathetic activity that accompanies exercise despite the seemingly contradictory fact that arterial pressure is higher than normal. In fact, were it not for the arterial baroreceptor reflex, the decrease in total peripheral resistance that occurs during exercise would cause mean arterial pressure to fall well below normal.
As discussed in Chapter 9 and indicated in Figures 10–4 and 10–5, cutaneous blood flow may increase during exercise despite a generalized increase in sympathetic vasoconstrictor tone because thermal reflexes can override pressure reflexes in the special case of skin blood flow control. Temperature reflexes, of course, are usually activated during strenuous exercise to dissipate the excess heat being produced by the active skeletal muscles. Often cutaneous flow decreases at the onset of exercise (as part of the generalized increase in arteriolar tone from increased sympathetic vasoconstrictor activity) and then increases later during exercise as body heat builds up.
In addition to the increases in the skeletal muscle and skin blood flow, coronary blood flow increases substantially during strenuous exercise. This is primarily due to local metabolic vasodilation of coronary arterioles as a result of increased cardiac work and myocardial oxygen consumption.
Two important mechanisms that participate in the cardiovascular response to dynamic exercise are not shown in Figure 10–5. The first is the skeletal muscle pump, which was discussed in connection with upright posture. The skeletal muscle pump is a very important factor in promoting venous return during dynamic exercise preventing the reflex-induced increase in cardiac output from drastically lowering central venous pressure. The second factor is the respiratory pump, which also promotes venous return during exercise. Exaggerated respiratory movements that occur during exercise increase the effectiveness of the respiratory pump and thus enhance venous return and cardiac filling.
As indicated in Figure 10–4, the average central venous pressure does not change much, if at all, during strenuous dynamic exercise. This is because the cardiac output and the venous return curves are both shifted upward during exercise. Therefore, the cardiac output and venous return will be elevated without a significant change in central venous pressure. Thus, the increase in stroke volume that accompanies exercise (suggested in this figure by the increase in pulse pressure) largely reflects the increased myocardial contractility and increased ejection fraction with decreased end-systolic ventricular volume.
![]() In summary, the profound cardiovascular adjustments to dynamic exercise shown in Figure 10–5 all occur automatically as a consequence of the operation of the normal cardiovascular control mechanisms. The tremendous increase in skeletal muscle blood flow is accomplished largely by increased cardiac output but also in part by diverting flow away from the kidneys and the splanchnic organs.
In summary, the profound cardiovascular adjustments to dynamic exercise shown in Figure 10–5 all occur automatically as a consequence of the operation of the normal cardiovascular control mechanisms. The tremendous increase in skeletal muscle blood flow is accomplished largely by increased cardiac output but also in part by diverting flow away from the kidneys and the splanchnic organs.
Static exercise (ie, isometric) presents a much different disturbance on the cardiovascular system than does dynamic exercise. As discussed in the previous section, dynamic exercise produces large reductions in total peripheral resistance because of local metabolic vasodilation in exercising muscles. Static efforts, even of moderate intensity, cause a compression of the vessels in the contracting muscles and a reduction in the blood flow through them. Thus, total peripheral resistance does not usually fall during strenuous static exercise and may even increase significantly. The primary disturbances on the cardiovascular system during static exercise seem to be set point–raising inputs to the medullary cardiovascular centers from the cerebral cortex (central command) and from chemoreceptors and mechanoreceptors in the contracting muscle. These inputs result in another example of what is termed the “exercise pressor response.”
Cardiovascular effects of static exercise include increases in the heart rate, cardiac output, and arterial pressure—all of which are the result of increases in sympathetic drive. Static exercise, however, produces less of an increase in the heart rate and cardiac output and more of an increase in diastolic, systolic, and mean arterial pressure than does dynamic exercise. Because of the higher afterload on the heart during static exercise, cardiac work is significantly higher than during dynamic exercise.
The time course of recovery of the various cardiovascular variables after a bout of exercise depends on many factors, including the type, duration, and intensity of the exercise as well as the overall fitness of the individual. Muscle blood flow normally returns to a resting value within a few minutes after dynamic exercise. However, if an abnormal arterial obstruction prevents a normal active hyperemia from occurring during dynamic exercise, the recovery will take much longer than normal. After isometric exercise, muscle blood flow often rises to near-maximum levels before returning to normal with a time course that varies with the duration and intensity of the effort. Part of the increase in muscle blood flow that follows isometric exercise might be classified as reactive hyperemia in response to the blood flow restriction caused by compressional forces within the muscle during the exercise.
Responses to Chronic Exercise
Physical training or “conditioning” produces substantial beneficial effects on the cardiovascular system. The specific alterations that occur depend on the type of exercise, the intensity and duration of the training period, the age of the individual, and his or her original level of fitness.
![]() In general, however, repeated physical exercise over a period of several weeks is associated with an increase in the individual’s work capacity. Cardiovascular alterations associated with conditioning may include decreases in heart rate, increases in cardiac stroke volume, and decreases in arterial blood pressure at rest. During exercise, a trained individual will be able to achieve a given workload and cardiac output with a lower heart rate and higher stroke volume than will be possible by an untrained individual. These changes produce a general decrease in myocardial oxygen demand and an increase in the cardiac reserve (potential for increasing cardiac output) that can be called on during times of stress. Much of the cardiovascular benefit of exercise conditioning can be attributed to a significant increase in circulating blood volume. This is triggered by the repetitive activation of the sympathetic nervous system during training, which promotes the renal fluid retention mechanisms.
In general, however, repeated physical exercise over a period of several weeks is associated with an increase in the individual’s work capacity. Cardiovascular alterations associated with conditioning may include decreases in heart rate, increases in cardiac stroke volume, and decreases in arterial blood pressure at rest. During exercise, a trained individual will be able to achieve a given workload and cardiac output with a lower heart rate and higher stroke volume than will be possible by an untrained individual. These changes produce a general decrease in myocardial oxygen demand and an increase in the cardiac reserve (potential for increasing cardiac output) that can be called on during times of stress. Much of the cardiovascular benefit of exercise conditioning can be attributed to a significant increase in circulating blood volume. This is triggered by the repetitive activation of the sympathetic nervous system during training, which promotes the renal fluid retention mechanisms.
Ventricular chamber enlargement often accompanies dynamic exercise conditioning regimens (endurance training), whereas increases in myocardial mass and ventricular wall thickness are more pronounced with static exercise conditioning regimens (strength training). These structural alterations improve the pumping capabilities of the myocardium. However, as described in the next chapter, ventricular chamber enlargement and myocardial hypertrophy are not always hallmarks of improved cardiac performance but may be adaptive responses to various pathological states that, if extreme, may not be helpful.
Exercise training or “conditioning” with a higher-than-normal blood volume represents the opposite end of a functional spectrum from the “deconditioning” effects of long-term bed rest with lower-than-normal blood volume. “Deconditioning” after cessation of an exercise program occurs rapidly as blood volume returns to resting levels.
It is clear that exercise and physical conditioning can significantly reduce the incidence and mortality of cardiovascular disease. Although studies have not established specific mechanisms for these beneficial effects, there is a positive correlation between physical inactivity and the incidence rate and intensity of coronary heart disease. It is increasingly evident that recovery from a myocardial infarction or cardiac surgery is enhanced by an appropriate increase in physical activity. The benefits of cardiac rehabilitation programs include improvement in various indices of cardiac function as well as improvements in physical work capacity, percent body fat, serum lipids, psychological sense of well-being, and quality of life.
NORMAL CARDIOVASCULAR ADAPTATIONS
Up to this point, the cardiovascular system of a healthy adult has been described. However, there are some important cardiovascular adaptations that accompany pregnancy, birth, growth, and aging. The material in the following section is a brief overview of these changes.
Maternal Cardiovascular Changes during Pregnancy
![]() Pregnancy causes alterations in vascular structure and blood flow to many maternal organs in order to support growth of the developing fetus. These organs include not only the uterus and developing placenta but also the kidneys and the gastrointestinal organs. However, one of the most striking cardiovascular changes of pregnancy is the nearly 50% increase in circulating blood volume. The placenta, being a low-resistance organ added in parallel with the other systemic organs, reduces the overall systemic total peripheral resistance by approximately 40%. Without the substantial increase in circulating blood volume to support cardiac filling, the necessary elevation in cardiac output to balance the decrease in total peripheral resistance would not be possible and pregnancy would result in a substantial decrease in mean arterial pressure. At birth, the loss of the placenta contributes to the return of maternal total peripheral resistance back toward normal levels.
Pregnancy causes alterations in vascular structure and blood flow to many maternal organs in order to support growth of the developing fetus. These organs include not only the uterus and developing placenta but also the kidneys and the gastrointestinal organs. However, one of the most striking cardiovascular changes of pregnancy is the nearly 50% increase in circulating blood volume. The placenta, being a low-resistance organ added in parallel with the other systemic organs, reduces the overall systemic total peripheral resistance by approximately 40%. Without the substantial increase in circulating blood volume to support cardiac filling, the necessary elevation in cardiac output to balance the decrease in total peripheral resistance would not be possible and pregnancy would result in a substantial decrease in mean arterial pressure. At birth, the loss of the placenta contributes to the return of maternal total peripheral resistance back toward normal levels.
Fetal Circulation and Changes at Birth
![]() During fetal development, the exchange of nutrients, gases, and waste products between fetal and maternal blood occurs in the placenta. This exchange occurs by diffusion between separate fetal and maternal capillaries without any direct connection between the fetal and maternal circulations. From a hemodynamic standpoint, the placenta represents a temporary additional large systemic organ for both the fetus and the mother. The fetal component of the placenta has a low vascular resistance and receives a substantial portion of the fetal cardiac output.
During fetal development, the exchange of nutrients, gases, and waste products between fetal and maternal blood occurs in the placenta. This exchange occurs by diffusion between separate fetal and maternal capillaries without any direct connection between the fetal and maternal circulations. From a hemodynamic standpoint, the placenta represents a temporary additional large systemic organ for both the fetus and the mother. The fetal component of the placenta has a low vascular resistance and receives a substantial portion of the fetal cardiac output.
Blood circulation in the developing fetus completely bypasses the collapsed fetal lungs. No blood flows into the pulmonary artery because the vascular resistance in the collapsed fetal lungs is essentially infinite (perhaps induced by the hypoxic status of the fetal alveoli). By the special structural arrangements shown in Figure 10–6, the right and left sides of the fetal hearts actually operate in parallel to pump blood through the systemic organs and the placenta. As shown in Figure 10–6A, fetal blood returning from the systemic organs and placenta fills both the left and right sides of the hearts together because of an opening in the intra-atrial septum called the foramen ovale. As indicated in Figure 10–6B, blood that is pumped by the right side of the fetal heart does not enter the occluded pulmonary circulation but is rather diverted into the aorta through a vascular connection between the pulmonary artery and the aorta called the ductus arteriosis.

Figure 10–6. Fetal circulation during (A) cardiac filling and (B) cardiac ejection. pv, pulmonary veins; la, left atrium; lv, left ventricle; rv, right ventricle; ra, right atrium; vc, venae cavae; a, aorta; pa, pulmonary artery.
An abrupt decrease in pulmonary vascular resistance occurs at birth with the onset of lung ventilation. The sudden increase in alveolar oxygen causes pulmonary vasodilation. This permits blood to begin flowing into the lungs from the pulmonary artery and tends to lower pulmonary arterial pressure. Meanwhile, total systemic vascular resistance increases greatly because of loss of the placenta (which is a large organ with low vascular resistance). This causes a rise in aortic pressure, which retards or even reverses the flow through the ductus arteriosis. Through mechanisms that are incompletely understood but clearly linked to a rise in blood oxygen tension, the ductus arteriosis gradually constricts and completely closes over time, normally ranging from hours to a few days. The circulatory changes that occur at birth tend to simultaneously increase the pressure afterload on the left side of the heart and decrease that on the right. This indirectly causes left atrial pressure to increase above that in the right atrium so that the pressure gradient for flow through the foramen ovale is reversed. Reverse flow through the foramen ovale is, however, prevented by a flap-like valve that covers the opening in the left atrium. Normally, the foramen ovale is eventually closed permanently by the growth of fibrous tissue.
Pediatric Cardiovascular Characteristics
![]() Cardiovascular variables change significantly between birth and adulthood. The healthy neonate has, by adult standards, a high resting heart rate (average of 140 beats/min) and a low arterial blood pressure (average of 60/35 mm Hg). These average values rapidly change over the first year (to 120 beats/min and 100/65 mm Hg, respectively). By the time the child enters adolescence, these values are near adult levels.
Cardiovascular variables change significantly between birth and adulthood. The healthy neonate has, by adult standards, a high resting heart rate (average of 140 beats/min) and a low arterial blood pressure (average of 60/35 mm Hg). These average values rapidly change over the first year (to 120 beats/min and 100/65 mm Hg, respectively). By the time the child enters adolescence, these values are near adult levels.
Pulmonary vascular resistance decreases precipitously at birth, as described earlier, and then continues to decline during the first year, at which time pulmonary pressures resemble adult levels. These resistance changes appear to be due to a progressive remodeling of the microvascular arterioles from thick-walled, small-diameter vessels to thin-walled, large-diameter microvessels.
It is noteworthy that distinct differences between right and left ventricular mass and wall thickness develop only after birth. Presumably they arise because of a hypertrophic response of the left ventricle to the increased afterload it must assume at birth. Accordingly, the electrocardiogram of children shows an early right ventricular dominance (electrical axis orientation) that converts to the normal left ventricular dominance during childhood.
Heart murmurs are also quite common in childhood and have been reported to be present in as many as 50% of healthy children. Most of these murmurs fall in the category of “innocent” murmurs, resulting from normal cardiac tissue vibrations, high flow through valves, and thin chest walls that make noises from the vasculature easy to hear. Less than 1% of them result from various congenital heart defects.
Growth and development of the vascular system parallels growth and development of the body with most of the local and reflex regulatory mechanisms operational shortly after birth.
Cardiovascular Changes with Normal Aging
In general, as persons get older, they get slower, stiffer, and drier. Connective tissue becomes less elastic, capillary density decreases in many tissues, mitotic activity of dividing cells becomes slower, and fixed postmitotic cells (such as nerve and muscle fibers) are lost. Although these changes do not, in general, alter the basic physiological processes, they do have an influence on the rate at which various homeostatic mechanisms operate.
![]() Age-dependent changes that occur in the heart include (1) a decrease in the resting and maximum cardiac index, (2) a decrease in the maximum heart rate, (3) an increase in the contraction and relaxation time of the heart muscle, (4) an increase in the myocardial stiffness during diastole, (5) a decrease in the number of functioning myocytes, and (6) an accumulation of pigment in the myocardial cells.
Age-dependent changes that occur in the heart include (1) a decrease in the resting and maximum cardiac index, (2) a decrease in the maximum heart rate, (3) an increase in the contraction and relaxation time of the heart muscle, (4) an increase in the myocardial stiffness during diastole, (5) a decrease in the number of functioning myocytes, and (6) an accumulation of pigment in the myocardial cells.
Changes that occur in the vascular bed with age may include (1) a decrease in capillary density in some tissues, (2) a decrease in arterial and venous compliance, (3) endothelial dysfunction associated with an increase in total peripheral vascular resistance, (4) an increase in arterial pulse pressure, and (5) an increase in mean arterial pressure (as discussed in Figure 6–10 in Chapter 6). The increase in arterial pressure may impose a greater afterload on the heart, which may be partially responsible for the age-dependent decreases in cardiac index.
Arterial baroreceptor–induced responses to changes in blood pressure are blunted with age. This is due in part to a decrease in afferent activity from the arterial baroreceptors because of the age-dependent increase in arterial rigidity. In addition, the total amount of norepinephrine contained in the sympathetic nerve endings of the myocardium decreases with age, and the myocardial responsiveness to catecholamines declines. Thus, the efferent component of the reflex is also compromised. These changes may partially account for the apparent age-dependent sluggishness in the responses to postural changes and recovery from exercise.
It is important (although often difficult) to separate true age-dependent alterations from changes due to progressive inactivity or from disease-induced changes in physiological function. Cardiovascular diseases are the major cause of death in an aging population. Atherosclerosis and hypertension are the primary culprits in many populations. These “diseases” lack the universality necessary to be categorized as true aging processes but generally occur with increasing incidence in the older population. Pharmacological interventions and reduction of risk factors (smoking, obesity, inactivity, and high-fat or high-sodium diets) by modification of lifestyle can alter the incidence, intensity, and progression of these cardiovascular diseases. It is also possible that some of the previously mentioned interventions may prevent early expression of some of the normal aging processes and prolong the lifespan of a given individual. No practical intervention, however, is currently available that will increase the maximum potential lifespan of humans.
Gender Differences in Cardiovascular Characteristics
![]() There are a few well-documented gender-dependent differences in the cardiovascular system. Compared with age-matched men, premenopausal women have a lower left ventricular mass to body mass ratio, which may reflect a lower cardiac afterload in women. This may result from their lower arterial blood pressure, greater aortic compliance, and improved ability to induce vasodilatory mechanisms (such as endothelial-dependent flow-mediated vasodilation). These differences are thought to be related to protective effects of estrogen and may account for the lowered risk of premenopausal women for developing cardiovascular disease. After menopause, these gender differences disappear. In fact, older women with ischemic heart disease often have a worse prognosis than men.
There are a few well-documented gender-dependent differences in the cardiovascular system. Compared with age-matched men, premenopausal women have a lower left ventricular mass to body mass ratio, which may reflect a lower cardiac afterload in women. This may result from their lower arterial blood pressure, greater aortic compliance, and improved ability to induce vasodilatory mechanisms (such as endothelial-dependent flow-mediated vasodilation). These differences are thought to be related to protective effects of estrogen and may account for the lowered risk of premenopausal women for developing cardiovascular disease. After menopause, these gender differences disappear. In fact, older women with ischemic heart disease often have a worse prognosis than men.
There are also gender-dependent differences in cardiac electrical properties. Women often have lower intrinsic heart rates and longer QT intervals than do men. They are at greater risk of developing long-QT syndrome and torsades de pointes. They are also twice as likely as men to have atrioventricular nodal reentry tachycardias.
However, it should be noted that most basic cardiovascular physiological processes are not greatly influenced by gender, and that individual differences in physiological responses within genders are usually as large as, or larger than, differences between genders.
PERSPECTIVES
The ability of the cardiovascular system to adjust to the challenges of the normal life of a healthy individual is very impressive. In all situations, regulation of arterial pressure by changes in cardiac output and vascular resistance assures that blood flow to critical organs is properly regulated and matched to the metabolic needs of the individual cells.
One of the topics missing from this text has to do with maintenance and repair of the cardiovascular components. It is obvious that subcellular mechanisms exist throughout life that are very good at building new structures during growth and development, at adapting existing structures to the demands of everyday life, and at mending damaged parts of the cardiovascular system as they are exposed to daily external insults. Major advances in our knowledge of how to care for our cardiovascular system have contributed greatly to the improvement in our longevity and quality of life in the later years. However, we humans seem to have an approximate 100-year maximum limit on our lifespan. Theories of aging suggest that senescence is not totally left to chance or to the accidental accumulation of subcellular errors. Planned obsolescence seems to be a major part of most life forms, and personal longevity is partially controlled by genetics. An age-dependent progressive inability to rebuild and repair our cardiovascular system seems to be a major contributor to overall deterioration of all bodily functions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


