Current guidelines recommend an implantable cardioverter-defibrillator (ICD) according to the left ventricular ejection fraction (LVEF). However, they do not mandate volumetric LVEF assessment. We sought to determine whether volumetric LVEF measurement using cardiovascular magnetic resonance imaging (CMR-LVEF) is superior to conventional LVEF measurement using 2-dimensional transthoracic echocardiography (Echo-LVEF) for risk stratifying patients referred for primary prevention ICD. Patients who underwent primary prevention ICD implantation at our institution and had undergone preimplantation CMR-LVEF from November 2001 to February 2011 were identified. Volumetric CMR-LVEF was determined from cine short-axis data sets. CMR-LVEF and Echo-LVEF were extracted from the clinical reports. The end point was appropriate ICD discharge (shock and/or antitachycardia pacing). Of 48 patients, appropriate ICD discharge occurred in 9 (19%) within 29 ± 25 months (range 1 to 99, median 20). All patients met the Echo-LVEF criteria for ICD implantation; however 25% (95% confidence interval 13% to 37%) did not meet the CMR-LVEF criteria. None (0%) of these latter patients had received an appropriate ICD discharge. Using CMR-LVEF ≤30% as a threshold for ICD eligibility, 19 patients (40%) with a qualifying Echo-LVEF would not have been referred for ICD, and none (0%) received an ICD discharge.For primary prevention ICD implantation, volumetric CMR-LVEF might be superior to clinical Echo-LVEF for risk stratification and can identify a large minority of subjects in whom ICD implantation can be safely avoided. In conclusion, if confirmed by larger prospective series, volumetric methods such as CMR should be considered a superior “gatekeeper” for the identification of patients likely to benefit from primary prevention ICD implantation.
Current guidelines have resulted in many Medicare beneficiaries becoming eligible for primary prevention implantable cardioverter-defibrillator (ICD) implantation in the United States alone. In the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT), most patients with ICD implantation for primary prevention never received a device discharge, with the average rate of appropriate ICD shocks estimated at 5.1% annually. Moreover, there appears to be a group of patients with a low left ventricular (LV) ejection fraction (LVEF) who remain at low risk of sudden cardiac death, although predictive measures have not been well validated. Currently, 2-dimensional transthoracic echocardiography is the most commonly used clinical method for serial measurement of the LV volumes and LVEF, because it is noninvasive and widely available. Cardiovascular magnetic resonance imaging (CMR) is also noninvasive and well-suited for assessment of the LV volumes and LVEF. Volumetric CMR methods have been shown to be both highly accurate and highly reproducible for the measurement of LV volumes and LVEF and superior to biplane echocardiographic and biplane CMR methods. It has also been previously recognized that transthoracic echocardiography (Echo-LVEF) underestimates the LVEF measured using CMR (CMR-LVEF), especially in patients with poor LVEF, with better correlation of 3-dimensional echocardiography with CMR. Given that current guidelines recommend ICD implantation for patients with a poor LVEF, without mandating a volumetric method, we hypothesized that the difference between 2-dimensional and volumetric assessment of LVEF would have a high effect on decision making and the outcome of patients considered for ICD implantation. Thus, we sought to determine the effect of volumetric CMR-LVEF compared to Echo-LVEF for risk stratification of patients referred for ICD implantation.
Methods
In the present retrospective cohort study, the Beth Israel Deaconess Medical Center clinical CMR database was queried to identify all patients undergoing ICD implantation for primary sudden cardiac death prevention from November 2001 to February 2011, who also had undergone preimplantation CMR. The patient demographics and clinical follow-up records from the hospital electronic medical records were reviewed.
All patients with ischemic cardiomyopathy had a clinical Echo-LVEF of ≤30% or Echo-LVEF of ≤35% with New York Heart Association class II or III heart failure. All patients with nonischemic dilated cardiomyopathy had an Echo-LVEF of ≤35% with New York Heart Association class II or III.
The clinical CMR-LVEF was measured for each patient, although the data were not applied to determine ICD implantation eligibility.
CMR and transthoracic echocardiography (without any contrast agent) were performed within 1 week of each other; otherwise, the subject had to have a second echocardiogram performed either before or after CMR, with the 2 Echo-LVEF values in agreement (i.e., the LVEF from both studies had to be on the same side of the guidelines classification). If no other echocardiographic study were available, we determined whether any possible interfering clinical events, such as myocardial infarction, hospitalization for heart failure exacerbation, and ICD shock, had occurred.
The study was performed with institutional review board approval of the Beth Israel Deaconess Medical Center. Written informed consent was waived.
CMR was performed using a Philips 1.5T (Philips Medical Systems, Amsterdam, The Netherlands) CMR scanner with a commercial 5-element cardiac-surface coil. Cine images were acquired in contiguous LV short-axis orientation with an electrocardiography-gated, breath-hold, steady-state, free-precession cine sequence with full LV coverage (8-mm slice thickness, 2-mm interslice gap, in-plane spatial resolution 2 × 2 mm, and 30-ms temporal resolution).
The endocardial borders at end-diastole and end-systole were manually traced using standard system software analysis tools. The end-diastolic volume and end-systolic volume were computed by the summation of the disks method, in which the sum of the cross-sectional areas was multiplied by the slice thickness. The LVEF was computed as 100% × (end-diastolic volume − end-systolic volume)/end-diastolic volume. The CMR-LVEF data were extracted from the clinical reports.
Clinical transthoracic echocardiography was performed using standard methods for each patient, and the findings were used to determine eligibility for ICD implantation. The Echo-LVEF data were extracted from the clinical reports.
All ICDs were implanted using the standard surgical technique ; the choice of device was at the discretion of the implanting physician, and the device was activated at completion of implantation. The devices were programmed for both antitachycardial pacing and shock, with 3 zones of therapy, including shock for ventricular fibrillation, antitachycardial pacing followed by shock for fast ventricular tachycardia, and a monitored zone for slower ventricular tachycardia. The exact therapy settings were adjusted at the discretion of the implanting physician.
The ICDs were interrogated at 1 and 3 months after implantation and every 6 months thereafter in the device clinic, during which appropriate sensing was confirmed, the device was interrogated, and the recorded events and ICD discharges were reviewed. An appropriate ICD discharge was defined as antitachycardia pacing or shocks delivered for ventricular tachyarrhythmias.
Continuous variables are presented as the mean ± SD. Categorical variables are presented as numbers and percentages. The survival time for an appropriate ICD discharge outcome was defined as the interval (number of days) from ICD implantation to the appropriate ICD discharge. The end of the follow-up period was September 30, 2011. If the patient had not had an appropriate ICD discharge during the follow-up period, the patient’s outcome was considered censored. Univariate Cox regression analysis was used to assess the association between each variable of the baseline characteristics and the hazard function of the presence of an appropriate ICD discharge. The survival function of an appropriate ICD discharge was compared between the CMR-LVEF ≤30% and CMR-LVEF >30% cutoffs using Kaplan-Meier estimates and the log-rank test. All statistical analyses were performed using SAS, version 9.2 (SAS Institute, Cary, North Carolina). The type I error was set at 0.05.
Results
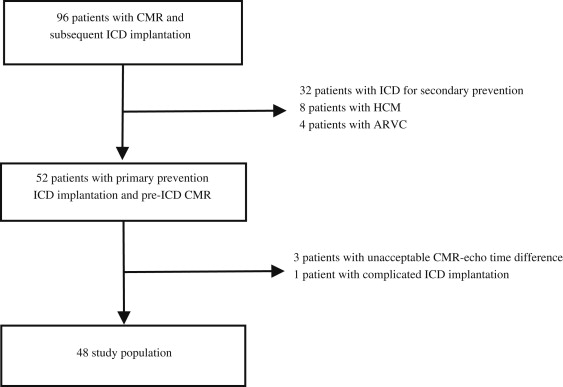
A total of 96 patients with CMR-LVEF data available before ICD implantation were identified, of whom 52 patients were referred for primary prevention of sudden cardiac death and underwent successful pre-ICD implantation CMR. Of these 52 patients, 3 had unacceptable CMR-Echo intervals and 1 had a complicated ICD implantation, which led to nonarrhythmic death 2 days after the procedure. These 4 patients were excluded from the analysis, leaving 48 patients in the present retrospective cohort study ( Figure 1 ). The mean follow-up period was 29 ± 25 months (range 1 to 99, median 20). The baseline characteristics of the entire cohort are summarized in Table 1 .
| Variable | All (n = 48) | Appropriate ICD Discharge | p Value | |
|---|---|---|---|---|
| Yes (n = 9) | No (n = 39) | |||
| Age (yrs) | 63 ± 11 | 68 ± 10 | 63 ± 11 | 0.678 |
| Men | 34 (71%) | 8 (89%) | 26 (67%) | 0.212 |
| Ischemic cardiomyopathy | 25 (52%) | 6 (67%) | 19 (49%) | 0.640 |
| Biventricular pacing | 15 (31%) | 2 (22%) | 13 (33%) | 0.237 |
| Diabetes | 16 (33%) | 6 (67%) | 10 (26%) | 0.052 |
| Hypertension | 36 (75%) | 7 (78%) | 29 (74%) | 0.892 |
| Dyslipidemia | 27 (56%) | 5 (56%) | 22 (56%) | 0.461 |
| β Blocker | 41 (85%) | 7 (78%) | 34 (87%) | 0.700 |
| Angiotensin-converting enzyme inhibitor | 45 (94%) | 9 (100%) | 36 (92%) | 0.995 |
| Antiarrhythmic | 2 (4%) | 0 | 2 (5%) | 0.994 |
| Aspirin | 37 (77%) | 7 (78%) | 30 (77%) | 0.730 |
| New York Heart Association class before cardioverter-defibrillator implantation | 2.4 ± 0.6 | 2.6 ± 0.8 | 2.5 ± 0.6 | 0.741 |
| Inappropriate implantable cardioverter-defibrillator discharge | 3 (6%) | 0 | 3 (8%) | 0.994 |
| Echocardiography–left ventricular ejection fraction (%) | 23 ± 6 | 19 ± 5 | 24 ± 7 | 0.018 |
| Cardiac magnetic resonance–left ventricular ejection fraction (%) | 29 ± 9 | 22 ± 6 | 31 ± 9 | 0.007 |
| Cardiac magnetic resonance–end-diastolic volume (ml) | 268 ± 88 | 301 ± 79 | 260 ± 90 | 0.237 |
| Cardiac magnetic resonance–end-diastolic volume index | 138 ± 40 | 153 ± 40 | 135 ± 39 | 0.200 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


