Based on the cardiovascular (CV) outcomes data derived predominantly from 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor (statin) trials, guidelines have set low-density lipoprotein (LDL) cholesterol targets at successively lower levels over time. Recent data have demonstrated that more-intensive statin therapy (and, consequently, lower LDL cholesterol level) is more effective at reducing CV events than less-intensive statin therapy. Although the average LDL cholesterol level for a United States adult is 119 mg/dl, within the “normal” range (90 to 130 mg/dl) per the United States National Cholesterol Education Program–Adult Treatment Panel III guidelines, data from fetal studies, diet studies, contemporary hunter-gatherer populations, and other mammals have suggested that the “normal” physiologic range for LDL cholesterol in humans is likely 50 to 70 mg/dl. Low LDL cholesterol levels have been sporadically associated with an increased risk of cancer, hemorrhagic stroke, and other complications in population studies and clinical trials. However, statin clinical trials have generally not demonstrated correlations between on-treatment LDL cholesterol levels and safety. Clinical data have suggested a linear relation between LDL cholesterol lowering and CV risk reduction, supporting a favorable risk/benefit ratio for attaining very low levels of LDL cholesterol to minimize the risk of CV events. In conclusion, clinical trial evidence demonstrating the efficacy and safety of LDL cholesterol lowering to a very low level is essential to ascertain the benefits and risks in reducing the residual risk of vascular disease.
Hypercholesterolemia is an established risk factor for atherosclerosis and the development of coronary heart disease (CHD). Early pivotal statin trials reported reductions in cardiovascular (CV) events by approximately 25% to 35% with reductions in baseline low-density lipoprotein (LDL) cholesterol levels from approximately 120 to 190 mg/dl to approximately 100 to 140 mg/dl. Data from recent statin trials have shown that more-aggressive lipid-lowering therapy is more effective at reducing CV events than less-aggressive lipid-lowering therapy. Still, doubt remains regarding the validity of the LDL cholesterol targets, with clinical trials of hypercholesterolemia designed to assess the efficacy and safety of different interventions (e.g., statin therapy vs placebo, more- vs less-intensive statin therapy) rather than to assess the merits and safety of achieving one specific LDL cholesterol level versus another. An important question that remains is whether the residual CV risk seen in these trials could be reduced or eliminated by further LDL cholesterol lowering. The present report reviewed the currently available clinical trial evidence that describes the effect of a very low level of LDL cholesterol (<50 mg/dl) in subjects at risk of CV events.
Human LDL Cholesterol Levels: Normal Versus Optimal
Data from the early period in life (from gestation to adolescence) and from populations that consume a non-Western diet have provided useful insight into what could be considered the “physiologic lipid levels” in humans. Total cholesterol and LDL cholesterol change as humans age ( Table 1 ). During late gestation in utero, the total cholesterol and LDL cholesterol levels reach approximately 55 and 30 mg/dl, respectively, and these levels increase during breastfeeding. A similar pattern has been observed in pigs and sheep though after weaning, the total cholesterol levels decrease dramatically in pigs and sheep but not in humans ( Figure 1 ). This phenomenon might result from the Western diet (as evidenced by comparisons of cholesterol levels across different populations and discussed by Dietschy and Turley, with data for nonhuman primates lending additional support to the effect of diet on serum lipids ). The cholesterol content of breast milk is believed to be sixfold greater than that of standard infant formula, and, accordingly, greater total cholesterol and LDL cholesterol levels have been described for breast-fed versus formula-fed infants. This greater cholesterol exposure in infancy has been postulated to downregulate cholesterol synthesis later in life. Data on cholesterol levels (LDL cholesterol levels not available) from the National Health and Nutrition Examination Surveys (NHANES III), focusing on children and adolescents aged 4 to 19 years (1988 to 1994), demonstrated a mean total cholesterol level of 165 mg/dl, with a peak level at ages 9 to 11 years (mean 171 mg/dl), a reduction at ages 12 to 15 years (mean 161 mg/dl), and a subsequent postpubertal increase at ages 16 to 19 years (mean 165 mg/dl). Similarly, the NHANES III data for children and adolescents (1999 to 2004) showed a mean total cholesterol concentration of 163.0 mg/dl among children and adolescents aged 6 to 17 years that peaked at around 8 to 10 years of age and then showed a postpubertal increase starting at 17 years of age.
| Age | Mean Total Cholesterol (mg/dl) | Mean LDL Cholesterol (mg/dl) |
|---|---|---|
| In utero (weeks) | ||
| 33–34 | 73 | 49 |
| 41–42 | 53 | 28 |
| Infancy (age 4–5 months ∗ ) | ||
| Breast fed | 183 | 83 |
| Formula fed | 112 | 48 |
| Children and adolescents (age 4–19 yrs, National Health and Nutrition Examination Survey 1988–1994) (yrs) | 165 | — |
| 4–5 | 162 | — |
| 6–8 | 166 | — |
| 9–11 | 171 | — |
| 12–15 | 161 | — |
| 16–19 | 165 | — |
| 12–19 | 163 | — |
| Adults (age 20–74 years, National Health and Nutrition Examination Survey 1999–2006) (yrs) | 200 | 119 |
| 20–39 | 189 | 113 |
| 40–59 | 209 | 124 |
| 60–74 | 209 | 123 |
∗ Based on a small series of breast-fed (n = 6) or formula-fed (n = 12) infants.
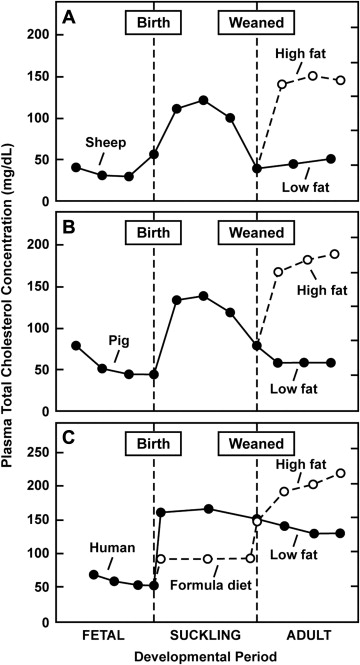
The contemporary Western diet appears to be a major contributor to the greater LDL cholesterol levels and atherosclerotic disease observed in Western populations. Subjects who consume a typical Western diet have an average total cholesterol level of about 200 mg/dl. This level, however, should not be considered normal, given the high rate of atherosclerosis in Western populations. According to the NHANES III data, the average serum LDL cholesterol and total cholesterol concentration among United States adults age 20 to 74 years (1999 to 2006) is 119 and 200 mg/dl, respectively. These levels are lower than previous NHANES-derived averages yet remain much greater than those documented in adult “wild-foraging” nonhuman primates (approximately 30 to 50 and 70 to 110 mg/dl, respectively). Westernized humans are the only adult mammals known to have a mean LDL cholesterol and total cholesterol level >80 and >160 mg/dl, respectively.
The human transition from a hunter-gatherer to an agricultural society is believed to underlie the observed change from a low-carbohydrate/high-protein diet, which included lean meat from game (with a high polyunsaturated fat content), to a greater consumption of grains and farm animal meat, which is higher in saturated fat. Today, the total cholesterol and LDL cholesterol levels of subjects from developed countries who do not consume red meat are lower than those of subjects who do consume red meat. A cross-sectional study performed in Brazil showed a mean LDL cholesterol level of 69, 88 to 101, and 123 mg/dl among subjects consuming vegan, vegetarian, and omnivorous diets, respectively (p <0.001 for omnivorous vs vegan diets). Similarly, many of the remaining hunter-gatherer societies have a LDL cholesterol and total cholesterol level of 70 and <135 mg/dl, respectively. McMurry et al showed that when Tarahumara Indians, whose traditional diet is low in fat and cholesterol and high in complex carbohydrates (e.g., corn, beans, and other vegetables, fruits, and small quantities of game, fish, and eggs), adopted a Western diet (high in fat, cholesterol, sugar, and calories), their mean LDL cholesterol levels increased 39%, from 72 to 100 mg/dl (p <0.001). Similarly, in a recent cross-sectional study, significantly greater levels of total cholesterol were observed in Intuit women and men, respectively, who were consuming a Western diet (with a high content of glycemic carbohydrates and saturated fat) versus a predominantly traditional Arctic marine diet (240.2 and 240.5 mg/dl vs 192.5 and 196.1 mg/dl; p <0.05 for a Western diet vs a traditional Arctic marine diet). In a prospective observational study initiated in Shanghai, China, during the 1970s, both the average baseline serum cholesterol level (4.2 mmol/L; 162.4 mg/dl) and the CHD mortality rate (7%) during the 8- to 13-year follow-up period were low; however, the baseline serum cholesterol was identified as a highly significant (p <0.001) predictor of CHD mortality. In summary, these reports suggest that “normal” Western levels of total cholesterol and LDL cholesterol are greater than those of our evolutionary ancestors and of those of non-Western human populations and Western vegetarians.
Safety of Very Low LDL Cholesterol Levels
Although these observations generally support the safety of lower LDL cholesterol levels than specified in the current treatment guidelines from North America, Europe, and Australia/New Zealand, some epidemiologic and clinical trial data have led to concern that very low levels of LDL cholesterol might increase the risk of cancer, hemorrhagic stroke, and non-CV death.
Data from the Framingham Study were used to examine the relation between lipoprotein levels and non-CHD outcomes, noted by the investigators as being limited by the small number of cases during the 6-year follow-up period. Logistic regression analyses showed significant inverse associations between the LDL cholesterol levels and stroke (both hemorrhagic and nonhemorrhagic) in women, cancer deaths in men, and death from non-CHD causes in both genders. Although the limited nature of the data precluded any conclusions regarding the risks of attaining low cholesterol levels, it brought to light the need for additional research to elucidate the relation between lipoprotein levels and outcomes beyond CHD and CHD-related mortality.
The relation between cholesterol levels and cancer incidence has been extensively investigated. In an early prospective study of approximately 22,000 men aged 35 to 64 years, the cholesterol levels did not differ between men with and men without, a cancer diagnosis during the ≥5-year follow-up period. However, a significantly lower cholesterol level was observed among patients diagnosed with cancer relative to the noncancer controls, specifically, within 2 years of cholesterol screening, a finding suggested by the study investigators as being a metabolic consequence of the underlying cancer. Two meta-analyses published during the 1990s found no inverse association between cholesterol levels and the overall risk of cancer or cancer-related mortality. These results are consistent with the conclusions of other analyses in which an association between cholesterol and cancer was either absent or was attributable to preclinical cancer.
Reports have been published suggesting that the effects of cholesterol levels on cancer risk are not neutral, including 1 study in which low cholesterol levels were not associated with an increased risk of total cancer or major site cancers, except for liver cancer (even after adjustment for early incident cases). That same study found an association between low cholesterol levels and a reduced risk of prostate cancer. In contrast, data from the Prostate Cancer Prevention Trial suggested that this relation might be restricted to the development of high-grade disease. Although the mechanism by which malignancy might lower cholesterol levels has not been fully elucidated to date, there are several possibilities, including the potential effect of tumor necrosis factor on cholesterol metabolism and a propensity for malignant cells to exhibit high levels of LDL receptor activity (and, consequently, enhanced LDL cholesterol clearance).
An accumulated body of data on the topic of statin use and cancer risk derived from other meta-analyses of clinical trials and observational studies have suggested that statins do not increase the overall risk of cancer, might potentially be protective against certain malignancies, and might be associated with reduced cancer-related mortality. The largest and most inclusive of these meta-analyses, the Cholesterol Treatment Trialists’ (CTT) Collaboration, analyzed data from 170,000 subjects in 26 randomized trials evaluating standard statin therapy versus controls (usual care/no treatment/placebo) and more-intensive versus less-intensive statin therapy. No significant differences were observed in cancer incidence or deaths from cancer between statin or more-intensive therapy versus control or less-intensive therapy. Furthermore, no indication was found that a reduction of LDL cholesterol in patients with a lower baseline LDL cholesterol level increased their cancer risk. Similarly, a recent meta-analysis of 27 large-scale, placebo-controlled statin trials and 5 trials of more-intensive versus less-intensive statin therapy showed no effect of statin therapy versus placebo or more-intensive versus less-intensive statin therapy on newly diagnosed cancer or cancer mortality. Overall, the CTT meta-analyses suggested that no evidence exists of an increased cancer incidence among statin users or an excess risk of cancer associated with more-intensive regimens versus standard regimens. Additionally, the most recent cancer site-specific analyses from the CTT group found no evidence of risk reduction for any specific cancer types (including prostate cancer and gastrointestinal malignancies) after a 5-year median follow-up period.
Concerns that low cholesterol might precipitate hemorrhagic stroke or be associated with an increase in CV-related mortality have stemmed from a small number of clinical trial observations. In the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial, a significant excess of hemorrhagic stroke, accompanied by a significant reduction in ischemic stroke and major vascular events, was observed with atorvastatin (vs placebo) among patients who had a recent history of a stroke or transient ischemic attack. On multivariate analysis, the risk of hemorrhagic stroke was significantly increased among patients with hypertension at the study visit preceding the stroke (hazard ratio [HR] 6.19, 95% confidence interval [CI] 1.47 to 26.11; p = 0.01); however, no relation was found between hemorrhagic stroke and baseline LDL cholesterol or the most recent on-treatment LDL cholesterol. The Treating to New Targets (TNT) study revealed a nonsignificant (p = 0.06) excess of non-CV deaths in the patients receiving atorvastatin 80 mg versus atorvastatin 10 mg, but no significant increase was seen in adverse events of any type in patients with an on-treatment LDL cholesterol level of ≤70 mg/dl versus those with higher LDL cholesterol levels. In the recent CTT meta-analysis, no indication was found that a reduction in the LDL cholesterol level in patients with lower baseline LDL cholesterol levels increased nonvascular mortality. An excess of hemorrhagic stroke in patients receiving statin therapy (vs placebo) or more-intensive statin therapy (vs less-intensive statin therapy) was not statistically significant (257 vs 220 events; p = 0.2).
Also of note, the rate of physician-reported diabetes was more frequent with rosuvastatin than with placebo (p = 0.01) in the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) study. However, because the rates of reported diabetes were not significantly different among the rosuvastatin-allocated participants with and without an LDL cholesterol level <50 mg/dl, the observed increase in new-onset diabetes appeared to be a non–LDL-related issue. Accordingly, data from a recent meta-analysis of large statin trials have suggested a slightly increased risk of incident diabetes in patients receiving statin therapy (9% increased risk, 95% CI 2% to 17%), and a change in LDL cholesterol levels did not account for the observed variation in the risk of diabetes. Results from another recent meta-analysis indicated that the risk of diabetes was greater with more-intensive statin therapy than with less-intensive statin therapy (12% increased risk, 95% CI 4% to 22%). A post hoc analysis of the data from the JUPITER trial suggested that the risk of developing diabetes appears to be limited to those patients already at high risk of developing diabetes (i.e., patients with impaired fasting glucose, the metabolic syndrome, severe obesity, or elevated hemoglobin A1c levels). Thus, the increased incidence of some adverse events observed in statin-treated patients did not correlate with the on-treatment LDL cholesterol levels and might not result from, per se, the achieved LDL cholesterol levels.
Subjects with genetic variations such as familial hypobetalipoproteinemia (FHBL) should also be considered when evaluating the safety of very low LDL cholesterol levels. FHBL is most commonly caused by APOB truncating mutations, with the heterozygous form (more common and extensively studied than the homozygous form) occurring at an estimated frequency of 1/500 to 1/1,000 in Western populations. Those with heterozygous FHBL are recognized as having a less severe lipoprotein deficiency (LDL cholesterol ∼30 to 50 mg/dl and total cholesterol ∼90 to 140 mg/dl) relative to those who are homozygous (or compound heterozygous) for FHBL-causing APOB mutations, associated with extremely low to undetectable levels of apolipoprotein B (apoB) and very low levels of LDL cholesterol (∼0 to 20 mg/dl) and total cholesterol (25 to 75 mg/dl). Although most heterozygotes are clinically asymptomatic, the clinical phenotype of FHBL homozygotes is highly variable, with null-allele homozygotes producing no detectable apoB and often having prominent clinical features, and “normotriglyceridemic” homozygotes possibly producing small amounts of apoB and could be asymptomatic. Because apoB is critical for the absorption of fat and fat-soluble vitamins in the intestine, the most severe cases have clinically evident fat and fat-soluble vitamin malabsorption and related disorders. Those with FHBL (predominantly heterozygotes for APOB mutations) might be at an increased risk of fatty liver disease (for which the underlying mechanism appears to be related to impaired hepatic secretion of low-density lipoproteins ); however, the long-term sequelae are not known. FHBL might provide protection against the development of coronary artery disease and, possibly, a longer than average life expectancy.
FHBL can also be caused by loss-of-function mutations in the proprotein convertase subtilisin/kexin type 9 ( PCSK9 ) gene. PCSK9 is a circulating protein that inhibits clearance of LDL cholesterol by binding to hepatic LDL receptors and facilitating their degradation. In the Atherosclerosis Risk In Communities (ARIC) prospective cohort study, PCSK9 nonsense mutations were identified in 2.6% of black participants and a PCSK9 sequence variation was identified in 3.2% of white participants. During the 15-year follow-up period, PCSK9 nonsense mutation carriers were associated with significant reductions in mean LDL cholesterol at baseline (28%; p <0.001) and in the risk of CHD (HR 0.11, 95% CI 0.02 to 0.81; p = 0.03) relative to noncarriers. Similarly, PCSK9 sequence variation mutation carriers were associated with significant reductions in mean LDL cholesterol at baseline (15%; p <0.001) and in the risk of CHD (HR 0.50, 95% CI 0.32 to 0.79; p = 0.003) relative to noncarriers. These 15-year observations suggest that the “residual” risk observed in 5-year clinical trials might be dramatically lowered with longer treatment periods. Patients with PCSK9 loss-of-function mutations are apparently healthy (1 patient was a 32-year-old black woman who was heterozygous for loss-of-function mutations in PCSK9 and the other was a 49-year-old white man who was heterozygous for 2 PCSK9 missense mutations; both patients were fully deficient in PCSK9) and have extremely low levels of LDL cholesterol (typically <20 mg/dl), without an increased risk of fatty liver disease. Those who are compound heterozygotes for loss-of-function mutations in PCSK9 , despite having no immunodetectable circulating PCSK9 and strikingly low LDL cholesterol levels, have shown no apparent adverse health issues.
A separate, and less common, genetic entity than FHBL, abetalipoproteinemia, occurs at a frequency of <1/1,000,000 humans and is linked to mutations in the gene encoding microsomal transfer protein, which is required for assembly and secretion of apoB-containing lipoproteins in the liver and intestine. Consequently, these subjects have near or complete absence of apoB-containing lipoproteins (chylomicrons, very-low-density lipoprotein, and LDL), with associated severe symptoms from the malabsorption of dietary fat and fat-soluble vitamins. No currently available cholesterol-lowering regimen is capable of achieving levels of LDL cholesterol this low, believed to reflect the severity of abetalipoproteinemia-associated malabsorption.
Safety of Very Low LDL Cholesterol Levels
Although these observations generally support the safety of lower LDL cholesterol levels than specified in the current treatment guidelines from North America, Europe, and Australia/New Zealand, some epidemiologic and clinical trial data have led to concern that very low levels of LDL cholesterol might increase the risk of cancer, hemorrhagic stroke, and non-CV death.
Data from the Framingham Study were used to examine the relation between lipoprotein levels and non-CHD outcomes, noted by the investigators as being limited by the small number of cases during the 6-year follow-up period. Logistic regression analyses showed significant inverse associations between the LDL cholesterol levels and stroke (both hemorrhagic and nonhemorrhagic) in women, cancer deaths in men, and death from non-CHD causes in both genders. Although the limited nature of the data precluded any conclusions regarding the risks of attaining low cholesterol levels, it brought to light the need for additional research to elucidate the relation between lipoprotein levels and outcomes beyond CHD and CHD-related mortality.
The relation between cholesterol levels and cancer incidence has been extensively investigated. In an early prospective study of approximately 22,000 men aged 35 to 64 years, the cholesterol levels did not differ between men with and men without, a cancer diagnosis during the ≥5-year follow-up period. However, a significantly lower cholesterol level was observed among patients diagnosed with cancer relative to the noncancer controls, specifically, within 2 years of cholesterol screening, a finding suggested by the study investigators as being a metabolic consequence of the underlying cancer. Two meta-analyses published during the 1990s found no inverse association between cholesterol levels and the overall risk of cancer or cancer-related mortality. These results are consistent with the conclusions of other analyses in which an association between cholesterol and cancer was either absent or was attributable to preclinical cancer.
Reports have been published suggesting that the effects of cholesterol levels on cancer risk are not neutral, including 1 study in which low cholesterol levels were not associated with an increased risk of total cancer or major site cancers, except for liver cancer (even after adjustment for early incident cases). That same study found an association between low cholesterol levels and a reduced risk of prostate cancer. In contrast, data from the Prostate Cancer Prevention Trial suggested that this relation might be restricted to the development of high-grade disease. Although the mechanism by which malignancy might lower cholesterol levels has not been fully elucidated to date, there are several possibilities, including the potential effect of tumor necrosis factor on cholesterol metabolism and a propensity for malignant cells to exhibit high levels of LDL receptor activity (and, consequently, enhanced LDL cholesterol clearance).
An accumulated body of data on the topic of statin use and cancer risk derived from other meta-analyses of clinical trials and observational studies have suggested that statins do not increase the overall risk of cancer, might potentially be protective against certain malignancies, and might be associated with reduced cancer-related mortality. The largest and most inclusive of these meta-analyses, the Cholesterol Treatment Trialists’ (CTT) Collaboration, analyzed data from 170,000 subjects in 26 randomized trials evaluating standard statin therapy versus controls (usual care/no treatment/placebo) and more-intensive versus less-intensive statin therapy. No significant differences were observed in cancer incidence or deaths from cancer between statin or more-intensive therapy versus control or less-intensive therapy. Furthermore, no indication was found that a reduction of LDL cholesterol in patients with a lower baseline LDL cholesterol level increased their cancer risk. Similarly, a recent meta-analysis of 27 large-scale, placebo-controlled statin trials and 5 trials of more-intensive versus less-intensive statin therapy showed no effect of statin therapy versus placebo or more-intensive versus less-intensive statin therapy on newly diagnosed cancer or cancer mortality. Overall, the CTT meta-analyses suggested that no evidence exists of an increased cancer incidence among statin users or an excess risk of cancer associated with more-intensive regimens versus standard regimens. Additionally, the most recent cancer site-specific analyses from the CTT group found no evidence of risk reduction for any specific cancer types (including prostate cancer and gastrointestinal malignancies) after a 5-year median follow-up period.
Concerns that low cholesterol might precipitate hemorrhagic stroke or be associated with an increase in CV-related mortality have stemmed from a small number of clinical trial observations. In the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial, a significant excess of hemorrhagic stroke, accompanied by a significant reduction in ischemic stroke and major vascular events, was observed with atorvastatin (vs placebo) among patients who had a recent history of a stroke or transient ischemic attack. On multivariate analysis, the risk of hemorrhagic stroke was significantly increased among patients with hypertension at the study visit preceding the stroke (hazard ratio [HR] 6.19, 95% confidence interval [CI] 1.47 to 26.11; p = 0.01); however, no relation was found between hemorrhagic stroke and baseline LDL cholesterol or the most recent on-treatment LDL cholesterol. The Treating to New Targets (TNT) study revealed a nonsignificant (p = 0.06) excess of non-CV deaths in the patients receiving atorvastatin 80 mg versus atorvastatin 10 mg, but no significant increase was seen in adverse events of any type in patients with an on-treatment LDL cholesterol level of ≤70 mg/dl versus those with higher LDL cholesterol levels. In the recent CTT meta-analysis, no indication was found that a reduction in the LDL cholesterol level in patients with lower baseline LDL cholesterol levels increased nonvascular mortality. An excess of hemorrhagic stroke in patients receiving statin therapy (vs placebo) or more-intensive statin therapy (vs less-intensive statin therapy) was not statistically significant (257 vs 220 events; p = 0.2).
Also of note, the rate of physician-reported diabetes was more frequent with rosuvastatin than with placebo (p = 0.01) in the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) study. However, because the rates of reported diabetes were not significantly different among the rosuvastatin-allocated participants with and without an LDL cholesterol level <50 mg/dl, the observed increase in new-onset diabetes appeared to be a non–LDL-related issue. Accordingly, data from a recent meta-analysis of large statin trials have suggested a slightly increased risk of incident diabetes in patients receiving statin therapy (9% increased risk, 95% CI 2% to 17%), and a change in LDL cholesterol levels did not account for the observed variation in the risk of diabetes. Results from another recent meta-analysis indicated that the risk of diabetes was greater with more-intensive statin therapy than with less-intensive statin therapy (12% increased risk, 95% CI 4% to 22%). A post hoc analysis of the data from the JUPITER trial suggested that the risk of developing diabetes appears to be limited to those patients already at high risk of developing diabetes (i.e., patients with impaired fasting glucose, the metabolic syndrome, severe obesity, or elevated hemoglobin A1c levels). Thus, the increased incidence of some adverse events observed in statin-treated patients did not correlate with the on-treatment LDL cholesterol levels and might not result from, per se, the achieved LDL cholesterol levels.
Subjects with genetic variations such as familial hypobetalipoproteinemia (FHBL) should also be considered when evaluating the safety of very low LDL cholesterol levels. FHBL is most commonly caused by APOB truncating mutations, with the heterozygous form (more common and extensively studied than the homozygous form) occurring at an estimated frequency of 1/500 to 1/1,000 in Western populations. Those with heterozygous FHBL are recognized as having a less severe lipoprotein deficiency (LDL cholesterol ∼30 to 50 mg/dl and total cholesterol ∼90 to 140 mg/dl) relative to those who are homozygous (or compound heterozygous) for FHBL-causing APOB mutations, associated with extremely low to undetectable levels of apolipoprotein B (apoB) and very low levels of LDL cholesterol (∼0 to 20 mg/dl) and total cholesterol (25 to 75 mg/dl). Although most heterozygotes are clinically asymptomatic, the clinical phenotype of FHBL homozygotes is highly variable, with null-allele homozygotes producing no detectable apoB and often having prominent clinical features, and “normotriglyceridemic” homozygotes possibly producing small amounts of apoB and could be asymptomatic. Because apoB is critical for the absorption of fat and fat-soluble vitamins in the intestine, the most severe cases have clinically evident fat and fat-soluble vitamin malabsorption and related disorders. Those with FHBL (predominantly heterozygotes for APOB mutations) might be at an increased risk of fatty liver disease (for which the underlying mechanism appears to be related to impaired hepatic secretion of low-density lipoproteins ); however, the long-term sequelae are not known. FHBL might provide protection against the development of coronary artery disease and, possibly, a longer than average life expectancy.
FHBL can also be caused by loss-of-function mutations in the proprotein convertase subtilisin/kexin type 9 ( PCSK9 ) gene. PCSK9 is a circulating protein that inhibits clearance of LDL cholesterol by binding to hepatic LDL receptors and facilitating their degradation. In the Atherosclerosis Risk In Communities (ARIC) prospective cohort study, PCSK9 nonsense mutations were identified in 2.6% of black participants and a PCSK9 sequence variation was identified in 3.2% of white participants. During the 15-year follow-up period, PCSK9 nonsense mutation carriers were associated with significant reductions in mean LDL cholesterol at baseline (28%; p <0.001) and in the risk of CHD (HR 0.11, 95% CI 0.02 to 0.81; p = 0.03) relative to noncarriers. Similarly, PCSK9 sequence variation mutation carriers were associated with significant reductions in mean LDL cholesterol at baseline (15%; p <0.001) and in the risk of CHD (HR 0.50, 95% CI 0.32 to 0.79; p = 0.003) relative to noncarriers. These 15-year observations suggest that the “residual” risk observed in 5-year clinical trials might be dramatically lowered with longer treatment periods. Patients with PCSK9 loss-of-function mutations are apparently healthy (1 patient was a 32-year-old black woman who was heterozygous for loss-of-function mutations in PCSK9 and the other was a 49-year-old white man who was heterozygous for 2 PCSK9 missense mutations; both patients were fully deficient in PCSK9) and have extremely low levels of LDL cholesterol (typically <20 mg/dl), without an increased risk of fatty liver disease. Those who are compound heterozygotes for loss-of-function mutations in PCSK9 , despite having no immunodetectable circulating PCSK9 and strikingly low LDL cholesterol levels, have shown no apparent adverse health issues.
A separate, and less common, genetic entity than FHBL, abetalipoproteinemia, occurs at a frequency of <1/1,000,000 humans and is linked to mutations in the gene encoding microsomal transfer protein, which is required for assembly and secretion of apoB-containing lipoproteins in the liver and intestine. Consequently, these subjects have near or complete absence of apoB-containing lipoproteins (chylomicrons, very-low-density lipoprotein, and LDL), with associated severe symptoms from the malabsorption of dietary fat and fat-soluble vitamins. No currently available cholesterol-lowering regimen is capable of achieving levels of LDL cholesterol this low, believed to reflect the severity of abetalipoproteinemia-associated malabsorption.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


