Visceral Debranching with Repair of Descending Thoracic and Visceral Aortic Aneurysm
S. W. Ham
M. Sigman
Fred Weaver
Indications
Various operative approaches have been described regarding the optimal surgical management of thoracoabdominal aortic aneurysms (TAAAs) from conventional open repair to total endovascular repair, and a combination of both approaches known as the hybrid repair. Operative morbidity and mortality of open repair for descending thoracic aortic aneurysm and TAAA have improved significantly at high-volume centers over the past 20 years. Centers of excellence have achieved operative mortality of 4.6% to 10% for TAAA. Despite tremendous reduction in overall operative mortality and morbidity with conventional open repair, real-world operative mortality, renal dysfunction, and paraplegia remain significant based on the National Inpatient Sample.
With the widespread availability of thoracic endovascular devices, a hybrid approach to descending thoracic aortic aneurysm and TAAA has emerged as an alternative and potentially less morbid technique to conventional open repair. The hybrid technique utilizes open surgical debranching of the visceral/renal vessels followed by endovascular aneurysm exclusion. This is generally achieved using individual retrograde bypasses to the visceral/renal arteries using distal inflow sites such as the common iliac arteries or infrarenal aorta with subsequent endovascular exclusion of the aneurysmal aortic segment. This technique has been used to treat Crawford–Safi type I through V TAAA, with the caveat that type I and II aneurysms may require left subclavian artery debranching by either transposition or bypass to establish an adequate proximal landing zone for the endograft. Among the several putative advantages of the hybrid procedure are the avoidance of thoracotomy, single-lung ventilation, division of the diaphragm, avoidance of an aortic cross clamp, decreased incidence of paraplegia, and shorter visceral/renal warm ischemia times. Obviating the
need of an aortic cross-clamp offers a major advantage of the hybrid approach over conventional open repair, notably in patients with compromised functional cardiac reserve.
need of an aortic cross-clamp offers a major advantage of the hybrid approach over conventional open repair, notably in patients with compromised functional cardiac reserve.
The hybrid approach has been generally applied to patients who are at high risk for standard open repair. It offers a less invasive and potentially safer approach to patients who are poor surgical candidates due to marginal cardiac and pulmonary function. Some centers have used the Society of Vascular Surgery (SVS) risk score to stratify operative risk and offer those with prohibitive perioperative risk a hybrid approach over open repair. Therefore the hybrid approach may be appropriately indicated in patients with adequate cardiac function but have severe pulmonary compromise and are unlikely to tolerate a two-cavity incision. Other factors including prior aortic procedures, prior thoracotomy, renal insufficiency, and those at high risk for paraplegia may also be considered for a hybrid repair.
Institutional reports have shown that aortic debranching, followed by endovascular aneurysm exclusion, is effective and feasible for the treatment of TAAA. Perioperative mortality rates, ranging from 2% to 24% and neurologic complications (spinal cord ischemia) ranging from 0% to 8.4% have been reported and in some reports are superior to traditional open repair. Recently, selected centers have reported the preferential use of the hybrid repair in low-risk patients, because of its imputed advantages. Our current practice is the preferential use of the hybrid procedure for the treatment of TAAA, reserving open repair for patients with connective tissue disorders or younger patients with atherosclerotic TAAA who have a low-risk profile.
Some authors have commented that most reports comparing a hybrid approach with conventional open surgery are small, use historic control groups for open surgery, and are inadequately powered to reliably detect differences. Some groups attribute higher than expected morbidity and mortality rates in hybrid cases to the high-risk patient population that typically undergoes this approach. In our experience, the hybrid approach confers benefit, in regard to minimizing rates of paraplegia, stroke, and renal dysfunction, however overall complication rates may be significant which underscores the importance of appropriate patient selection.
Contraindications
Contraindications to the hybrid approach include those common to conventional endovascular aortic aneurysm repair including inadequate proximal and distal landing zones. Furthermore, severe occlusive disease of the distal aorta or common iliac arteries precludes using these sites as inflow for visceral/renal bypasses. Small iliac artery diameter for device delivery is not a contraindication to the hybrid approach since a separate delivery conduit can be created by an anastomosis of a Dacron graft, at least 10 cm, to the contralateral iliac artery. The conduit is then tunneled in the retroperitoneum to the groin and sewn to the femoral artery. Femoral artery exposure is achieved through a modest transverse/oblique incision. It is then available for the TEVAR procedure. For patients with connective tissue disorders in whom a high likelihood exists for further aneurysmal degeneration (e.g., Marfan syndrome), endografting remains contraindicated in this patient population unless used as a bridge to definitive surgical repair.
Generally, preoperative cardiac evaluation for coronary artery or valvular disease is required. For most patients this includes a transthoracic echocardiogram and cardiac stress evaluation. Selective pulmonary evaluation may be required in patients with COPD associated respiratory compromise.
Site of Inflow Considerations
Preoperative planning considerations for the endovascular portion of the hybrid procedure are similar to those of standard TEVAR and are detailed in Chapters 31 and 32. Preoperative CT angiograms are required for appropriate endograft sizing but are also
extremely useful in the planning for a surgical debranching procedure. It allows assessment of any existing occlusive disease and extent of the aneurysmal disease which is critical in selecting sites of inflow and distal target vessels that will require bypass. Furthermore, this will largely be based on the patient’s individual anatomy and any previous abdominal aortic surgery. In patients who have undergone prior abdominal aortic surgery, the existing infrarenal aortic graft or the limb of previously placed aortobifemoral graft can be used for inflow.
extremely useful in the planning for a surgical debranching procedure. It allows assessment of any existing occlusive disease and extent of the aneurysmal disease which is critical in selecting sites of inflow and distal target vessels that will require bypass. Furthermore, this will largely be based on the patient’s individual anatomy and any previous abdominal aortic surgery. In patients who have undergone prior abdominal aortic surgery, the existing infrarenal aortic graft or the limb of previously placed aortobifemoral graft can be used for inflow.
If the infrarenal aorta will be used for inflow, there must be at least a 2-cm distance of normal aorta between the graft inflow site and the lowest part of the aneurysmal aortic segment to ensure proper distal sealing of the endograft and inadvertent coverage of the graft orifice. Similarly, when inflow is provided by the common iliac artery, at least 2 cm of normal aorta between the aortic bifurcation and lowest part or the aneurysmal aorta must be present as well. If the aneurysmal disease extends to the aortic bifurcation, the external iliac artery may be used as the inflow thereby allowing endovascular aneurysm exclusion using conventional devices available for EVAR. The main body of the EVAR device can be used as a platform for proximal extension using available thoracic endografts to treat the thoracic aneurysmal component.
The celiac artery, superior mesenteric artery (SMA), and bilateral renal arteries should be bypassed when the stent graft is anticipated to occlude their origin. However, the exact number of visceral/renal vessels that require bypass depends on the extent of aneurysmal disease, anticipated length of aortic coverage with the endograft, and presence of adequate seal zones.
Routine left subclavian artery revascularization is performed by carotid–subclavian artery transposition or bypass (although we prefer transposition) in cases where it is anticipated that the endograft will need to cover the left subclavian artery origin in order to achieve an adequate seal. The decision for preoperative lumbar spinal drain as a measure to prevent spinal cord ischemia is selective and generally surgeon dependent but factors to consider include history of prior aortic surgery, anticipated length of aortic coverage, and patency of hypogastric arteries.
Patients should receive a dose of prophylactic antibiotics 30 to 60 minutes prior to incision. A staged hybrid procedure with initial aortic debranching followed by an interval of patient recovery occurs prior to endovascular repair at a separate setting. Single-stage hybrid repairs are reserved for emergent presentations where endovascular aneurysm exclusion cannot be delayed.
Positioning
General anesthesia is used for all cases. The patient is then positioned according to the selected surgical approach to the abdominal aorta. Both transperitoneal and retroperitoneal approaches are feasible, with choice of approach largely dictated by surgeon preference, experience, and patient factors. For example, a retroperitoneal approach through a left oblique incision may be preferable for patients with prior transperitoneal operations, which allows dissection in a fresh plane and avoiding intraperitoneal scar tissue. It has been our general preference to use a left retroperitoneal approach using a oblique incision which provides excellent aortic exposure and access to all visceral/renal vessels. The one caveat to this preference is the position of the right renal artery with respect to the aneurysm and position of the renal origin on the aorta. In general, in patients in which the aneurysm involves the right renal artery or the origin of the renal artery is from 6 to 9 o’clock on a transverse cut of the aorta, a transperitoneal approach is preferred. This facilitates exposure and visualization of the distal renal anastomosis which is technically very challenging to almost impossible through a retroperitoneal approach.
Exposure
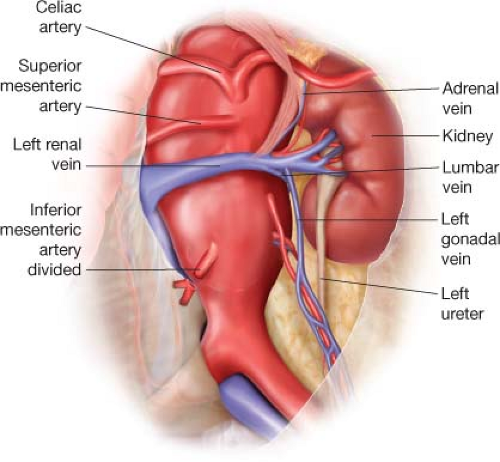
For the left retroperitoneal approach, the patient is placed supine with a bump placed under the left flank. The chest, abdomen, and groins and are prepped to the mid thighs and draped in standard surgical fashion. A oblique incision is made from the 10th interspace at the posterior axillary line to 1 cm below the umbilicus (Fig. 2.1). The anterior rectus sheath is incised and the rectus muscle divided. The posterior rectus sheath is incised sharply until peritoneum is visualized. The peritoneum is bluntly dissected off the posterior rectus sheath and lateral abdominal wall muscles while reflecting the underlying intra-abdominal contents anteromedially. The retroperitoneal dissection is carried out posteriomedially until the left kidney is encountered. The avascular plane between the peritoneum and the Gerota fascia of the left kidney is entered, which allows the left kidney and ureter to be kept in the renal fossa. The dissection is continued toward the aorta, sweeping the peritoneum and underlying bowel anteromedially. The inferior mesenteric artery is identified and ligated just beyond the aortic origin. This allows exposure of the infrarenal aorta and iliac vessels (Fig. 2.2). If inflow will be provided by the iliac vessels,
the right common iliac is preferred although if diseased, the left should be used. The left ureter should be identified as it crosses the left common iliac artery to prevent injury.
the right common iliac is preferred although if diseased, the left should be used. The left ureter should be identified as it crosses the left common iliac artery to prevent injury.
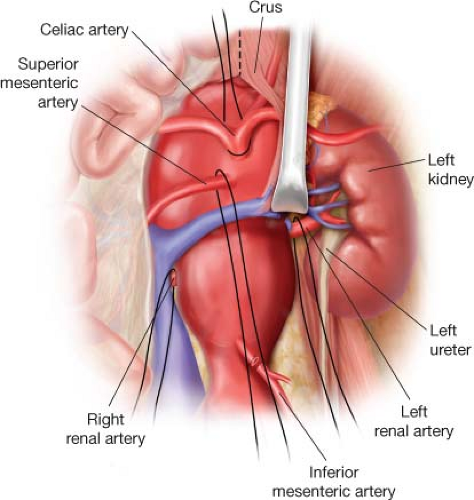 Figure 2.3 The diaphragmatic crus must be divided to adequately expose the celiac and superior mesenteric arteries at their origin. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree