Major Pathogens in Pulmonary Infections |
GENERAL PRINCIPLES
Viral infections of the upper and lower airways have a major impact on health. Acute respiratory illnesses, largely caused by viruses, are the most common illness experience for otherwise healthy adults and children (see Chapter 126). The National Health Interview Survey suggests that such illnesses are experienced at a rate of 85.6 illnesses per 100 persons per year, and account for 54% of all acute conditions exclusive of injuries.1 A total of 44% of these illnesses require medical attention, and result in 287 days of restricted activity, 94.4 days lost from work, and 182 days lost from school per 100 persons per year. Estimates from family-based surveillance suggest that approximately one-fourth of these illnesses result in consultation with a physician. Illness rates for all acute respiratory conditions are highest in young children; children below the age of 9 are estimated to experience between five and nine respiratory illnesses per year, whereas adults experience between three and five such illnesses.2,3
Mortality due to acute viral respiratory infection in otherwise healthy individuals in economically developed countries is relatively rare, with the exception of epidemic influenza. However, acute respiratory infection is a major cause of childhood mortality in developing countries, and it is estimated that 4.5 million children under 5 years of age die annually from acute respiratory infection.4 Viruses are identified in about 3% to 40% of cases of respiratory disease in this setting, and are estimated to play a contributing role in approximately 20% to 30% of deaths. In addition, new and emerging respiratory viruses such as Hantavirus, emerging coronaviruses associated with severe acute respiratory syndrome (SARS) or middle eastern respiratory syndrome (MERS), and transmission of influenza viruses from avian or swine sources to man, pose a continuing threat.
 EPIDEMIOLOGY
EPIDEMIOLOGY
Many of the viruses associated with acute respiratory disease display a significant seasonal variation in incidence. Although the exact seasonal arrival of each virus in the community cannot be predicted with precision, certain generalizations are useful diagnostically and in planning control strategies. For example, influenza and respiratory syncytial virus (RSV) epidemics both occur predominantly in the winter months, with a peak prevalence in January to March in the northern hemisphere. Parainfluenza virus type 3 (PIV-3) infections show a predominance in the spring, whereas types 1 and 2 (PIV-1 and PIV-2) cause outbreaks in the fall to early winter. Rhinoviruses may be isolated throughout the year, with increases in frequency in the spring and fall. The peak prevalence of enteroviral isolations is in late summer and early fall, whereas adenoviruses are isolated at roughly equal rates throughout the year. The herpes viruses also do not show significant seasonal variation in incidence, except for varicella, which occurs throughout the year, but more commonly in late winter and early spring.
The reasons for these seasonal changes are not entirely clear. One mechanism may involve seasonal effects on virus transmissibility either because of more favorable environmental conditions for virus survival5 or behavioral changes that increase transmission, such as indoor crowding. Heretofore unknown effects of season on host susceptibility or response to infection could also play a role.
 CHARACTERISTICS OF THE VIRUSES
CHARACTERISTICS OF THE VIRUSES
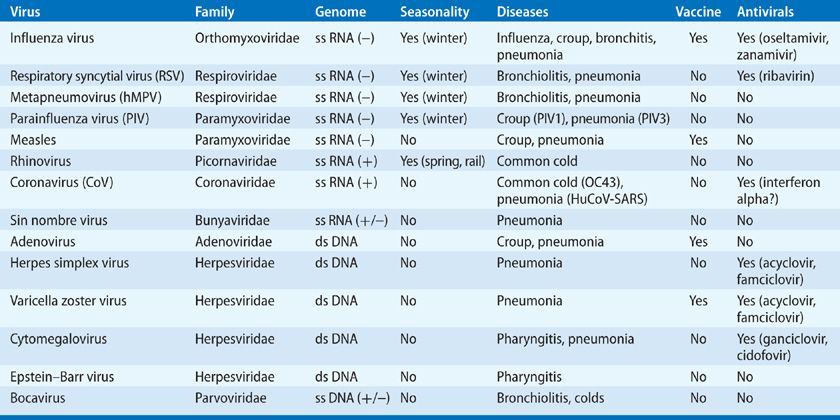
Viruses of importance in the respiratory tract include both those considered to be principal respiratory viruses, whose replication is generally restricted to the respiratory tract, and others in which respiratory involvement is part of a generalized infection. The classification of these viruses depends in part on the type and configuration of the nucleic acid in the viral genome, the characteristics of the viral structural proteins, and the presence or absence of a lipid-containing envelope surrounding the virus particle (Table 130-1). The number of distinct antigenic types in each of the virus families varies. For example, the adenovirus and rhinovirus groups are composed of large numbers of antigenically (serotypically) distinct immunotypes, but other groups, such as paramyxovirus and coronavirus, are composed of only a limited number of immunotypes. The degree of antigenic stability of the virus is an important factor in determining the frequency of reinfection. This characteristic is particularly important for influenza type A virus, which periodically undergoes both minor and major changes in its surface antigens.
TABLE 130-1 Common Respiratory Viruses
 TRANSMISSION
TRANSMISSION
The routes by which the different respiratory viruses spread from person to person are still not established with certainty. Rhinovirus and RSV spread, at least in part, by direct hand contact with contaminated skin and environmental surfaces. This is followed by self-inoculation of infectious virus onto the nasal mucosa or conjunctiva. Others, including influenza, measles, and varicella zoster viruses (VZV), spread at times in small-particle aerosols. Many viruses may spread by means of large-particle aerosols over short distances (1 m). The relative importance of the various transmission routes under natural conditions for each virus is unknown.
A number of respiratory viruses have been documented to cause outbreaks of infection in closed populations. In hospitals, nurseries, day care centers, and homes for the elderly, secondary spread to staff members and other patients may occur. Such outbreaks have been observed for viruses that appear to be spread by small-particle aerosols, including measles and VZV, and for those spread by direct contact with infectious secretions, such as RSV, rhinoviruses, and coronaviruses, where there is frequent close contact between patients and staff.
 PATHOGENESIS OF INFECTION
PATHOGENESIS OF INFECTION
The initial sites of infection and pathogenesis differ for the various virus groups. Some, such as rhinovirus, are associated mainly with upper respiratory tract involvement. Others, such as influenza, commonly invade the lower airways and sometimes pulmonary parenchyma in addition to causing upper airway disease. The viruses also differ in the amount of damage produced in the cells lining the respiratory tract. Extensive damage to the respiratory epithelium is a characteristic feature of the influenza virus infection, but biopsy studies show little evidence of nasal epithelial damage in persons with rhinovirus colds. Instead, colds are related both to virus damage to the respiratory tract and to the host responses to infection, including immunologic events, release of mediators of inflammation, and neurogenic reflexes.
An additional important feature of respiratory virus infections is their effect on the resident bacterial flora of the upper airways. Respiratory virus infections have been found to alter bacterial colonization patterns, increase bacterial adhesion to respiratory epithelium, and reduce mucociliary clearance and phagocytosis. These impairments of host defenses by virus allow colonization by pathogenic bacteria and invasion of normally sterile areas, such as the paranasal sinuses, middle ear, and lower respiratory tract, resulting in secondary infection.
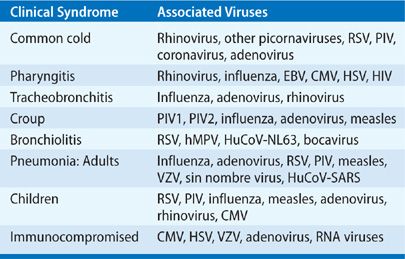
A summary of the specific viral etiologies most commonly associated with syndromes of upper respiratory tract infection is given in Table 130-2. These infections are discussed in detail in Chapter 126. The following sections briefly summarize the important points of viral respiratory tract infections.
TABLE 130-2 Virus-Associated Respiratory Tract Infections
 DIAGNOSTIC TESTS
DIAGNOSTIC TESTS
While specific diagnostic tests will be discussed in the appropriate sections, the development of molecular diagnostic techniques, particularly multiplexed nucleic acid–based tests, has generally supplanted most other diagnostic modalities. In most cases, however, diagnostic testing should be considered in the context of whether the results will have an impact on patient management.
THE COMMON COLD
Below are considered the clinical features, etiology, pathogenesis, diagnostic evaluation, and management of the common cold.
 CLINICAL FEATURES
CLINICAL FEATURES
The term “cold” really does not constitute a single entity, but rather a group of similar illnesses of differing cause (see also Chapter 126). However, all colds include symptoms of rhinitis with variable degrees of pharyngitis. Predominant associated symptoms include nasal stuffiness, sneezing, runny nose, and sore throat. Patients often report chills, but fever is not a typical feature of uncomplicated colds. Cough and hoarseness are variably present and may be more frequent in the elderly,6 but other lower respiratory tract signs and symptoms are not typical of colds and should raise suspicion of other entities.
Physical findings are nonspecific and most commonly include nasal discharge and pharyngeal inflammation. More severe disease, with higher fever, may be seen in children. Although colds are generally self-limited, symptoms may last for a surprisingly long period of time, with a median duration of illness of approximately 9 to 10 days in adults.7 Recognized complications of colds include secondary bacterial infections of the paranasal sinuses and middle ear, and exacerbations of asthma, chronic bronchitis, and emphysema. Colds are frequently associated with involvement of the middle ear, likely due to eustachian tube dysfunction. Colds are associated with symptomatic otitis media in approximately 2% of cases in adults, and in a higher proportion in young children.
Colds are also associated with detectable abnormalities of the paranasal sinuses, which may or may not be evident clinically. Mucosal thickening and/or sinus exudates have been observed in as many as 77% of subjects with acute colds.8 These abnormalities are transient and usually not associated with symptoms, although they may persist 21 days or longer. Colds are associated with symptomatic otitis media in approximately 2% of cases in adults,9 and in a higher proportion in young children.10 Rhinoviruses and other common cold viruses have been detected in middle ear fluids in approximately 20% to 40% of cases of otitis media with effusion in children.11 Infections with RSV, influenza, and adenoviruses are often also associated with otitis media.10 However, clinically manifest acute sinusitis is seen in a small (0.5%–5%) proportion of individuals with naturally occurring colds.
Clinical colds in atopic individuals may be more severe or more likely to result in wheezing than in normal individuals, and rhinoviruses have been identified as major causes of asthma exacerbations in children and adults.12 Rhinovirus colds may increase asthma by augmenting airway allergic responses such as histamine release and eosinophil influx after antigen challenge. Rhinoviruses have also been identified as important causes of exacerbations of chronic obstructive pulmonary disease (COPD).13,14
 VIRAL ETIOLOGIES AND DIFFERENTIAL DIAGNOSIS
VIRAL ETIOLOGIES AND DIFFERENTIAL DIAGNOSIS
Epidemiologic studies have established that the great majority of common colds are associated with infection with the human rhinoviruses or other picornaviruses. Other agents frequently associated with common colds include coronaviruses, PIV, and RSV, with a variety of other agents implicated occasionally. The clinical characteristics of illness due to each of these viruses are similar, and a specific viral etiology generally cannot be deduced on clinical grounds alone. Epidemiologic studies have indicated that on an annual basis, any one antigenic type of virus is responsible for less than 1% of all colds.
The differential diagnosis of individuals presenting with typical signs and symptoms is not extensive. However, in the presence of additional signs or symptoms that are not part of this clinical description, such as high persistent fever, signs of respiratory distress, or lower respiratory tract disease, alternative diagnoses should be sought. Allergic causes should be considered in individuals who present with recurrent symptoms restricted to the upper respiratory tract.
 PATHOGENESIS
PATHOGENESIS
Studies of the pathogenesis of the common cold have largely focused on rhinoviruses, the most commonly implicated viral etiology. In situ hybridization studies of nasal biopsy specimens from rhinovirus-infected subjects demonstrate that infection is largely confined to relatively small numbers of ciliated nasal mucosal epithelial cells, although occasional nonciliated cells are also infected.15 Sloughing of these epithelial cells is seen in naturally occurring colds, but the epithelial lining remains intact, with structurally normal cell borders. Infection is associated with significant increases in the numbers of polymorphonuclear leukocytes in nasal mucosa and secretions, probably due to elaboration of IL-8 by infected cells.16 Although rhinoviruses are not able to grow efficiently at body temperature, virus can be detected within cells of the lower airway even in uncomplicated colds in healthy subjects.17
In general, the number of infected cells appears to be quite limited, even in fairly symptomatic individuals, and there is no clear correlation between the level of virus replication or the number of cells infected, and the level of symptomatology. These results have suggested that virus-induced cellular injury is not the direct cause of symptoms in rhinovirus colds, but rather that inflammatory mediators play an important role. Analysis of the nature of the mucosal exudate during rhinovirus colds suggests that the nasal secretions during the initial response to rhinovirus infection are predominantly the result of increased vascular permeability, as demonstrated by elevated levels of plasma proteins in nasal secretions,18 whereas later glandular secretions (lactoferrin, lysozyme, and secretory IgA) predominate. Similar observations have been made in allergic rhinitis. However, in contrast to the situation in allergic rhinitis, histamine does not appear to play a role in the induction of symptoms in colds, as nasal histamine levels do not increase, and therapy with selective H1 antihistamine is not effective.19,20 Nasal secretion kinin levels do correlate with symptoms in natural and experimental colds, and intranasal administration of bradykinin mimics the induction of signs and symptoms in the common cold,20,21 including increased nasal vascular permeability, rhinitis, and sore throat.
 DIAGNOSTIC TESTS
DIAGNOSTIC TESTS
Molecular diagnostic tests have been developed for many of the viruses associated with the common cold. Since there is no specific therapy and the clinical characteristics of colds due to different viruses are similar, use of techniques for specific viral diagnosis in the common cold is generally limited to the research setting.
 TREATMENT AND PREVENTION
TREATMENT AND PREVENTION
The recommended treatment for colds is to use individual remedies to treat specific symptoms. Symptoms of sneezing and rhinorrhea can be alleviated with nonselective sedating antihistamines such as brompheniramine, chlorpheniramine, or clemastine fumarate.22,23 The effect is probably due to the anticholinergic properties of these drugs but treatment with selective H1 inhibitors is not effective. Studies of pseudoephedrine have demonstrated measurable improvements in nasal air flow consistent with a decongestant effect. In previously healthy children and adults, there is no danger from the routine use of cough suppressants, although they should be used cautiously in patients with underlying COPD. Cough syrups–containing expectorants are of unproved value in common colds, although guaifenesin may reduce the cough reflex.
Symptomatic therapy with systemic anticholinergic drugs or anticholinergic-sympathomimetic combinations has not been shown to confer any benefit, and to be associated with significant side effects, especially in children.24,25 In addition, the use of the decongestant phenylpropanolamine is associated with an increased risk of hemorrhagic stroke,17,26 and this drug has been removed from over-the-counter cold remedies.
Topical application of vasoconstrictors such as phenylephrine or ephedrine provides relief of nasal obstruction, but may be associated with a rebound of symptoms upon discontinuation if used for more than a few days. Thus, nasal sprays–containing decongestants should be used for no more than 3 days. Topical application of ipratropium, a quaternary anti-cholinergic agent that is minimally absorbed across biologic membranes, reduces rhinorrhea significantly in naturally occurring colds.27 This agent probably exerts its major effect on the parasympathetic regulation of mucus and seromucous glands.
There has been considerable interest in the development of antiviral agents for the common cold. Several problems confront the successful development of such an antiviral agent. Because of the numerous etiologic agents, the ideal drug would require a wide spectrum of activity. In addition, many drugs that appear to have excellent in vitro activity have failed in clinical trials, apparently because they did not reach sufficient levels within the nasal mucosa where virus replication occurs. Finally, because symptoms in colds are not clearly related to the level of virus replication, a successful treatment strategy may also require use of drugs to antagonize the effects of inflammatory mediators.28
LARYNGITIS AND PHARYNGITIS
In this section, the clinical features, etiology, differential diagnosis, pathogenesis, diagnostic evaluation, and management of laryngitis and pharyngitis are discussed.
 CLINICAL FEATURES
CLINICAL FEATURES
Pharyngitis is a common complaint of both adults and children, and is one of the more common reasons for seeking outpatient medical care. In general, this syndrome refers to individuals who present with the primary complaint of sore throat, and should probably be reserved for those individuals who manifest some objective evidence of pharyngeal inflammation as well. The clinical manifestations of pharyngitis are dominated by the specific causative agent. However, generally the syndrome can be divided into those cases in which nasal symptoms accompany pharyngitis, which are predominantly viral in nature, and those cases without nasal symptoms, which have a somewhat more diverse spectrum of etiologic considerations, including both group A and nongroup A streptococci, chlamydia (strain TWAR), mycoplasma, and other agents.29
 VIRAL ETIOLOGIES AND DIFFERENTIAL DIAGNOSIS
VIRAL ETIOLOGIES AND DIFFERENTIAL DIAGNOSIS
Rhinovirus colds are frequently accompanied by pharyngitis, although objective signs of pharyngeal inflammation are uncommon. Adenovirus infections are frequently associated with pharyngitis, and a specific syndrome of pharyngoconjunctival fever, consisting of fever, pharyngitis, and bilateral conjunctivitis is associated with adenovirus types 3 and 7. A variety of enteroviral serotypes are associated with febrile pharyngitis. Herpangina is a specific Coxsackie virus-induced pharyngitis in which small (1–2 mm) vesicular lesions of the soft palate rupture to become small white ulcers. Pharyngitis is a typical component of acute influenza in which individuals experience the sudden onset of systemic symptoms of fever, myalgias, and malaise accompanied by upper respiratory signs and symptoms, including pharyngitis. Primary oral infection with herpes simplex virus may present with pharyngitis, typically with vesicles and shallow ulcers of the palate, and cervical lymphadenopathy.
Pharyngitis may be the presenting or predominating symptom in more generalized viral infections. Pharyngitis is a significant complaint in approximately one-half of cases of the acute mononucleosis syndrome due to Epstein–Barr virus. Pharyngitis in this syndrome is generally exudative and is accompanied by cervical and generalized lymphadenopathy, as well as fever, hepatosplenomegaly, and other systemic symptoms. The heterophile antibody test is typically positive in the second week of illness. Cytomegalovirus (CMV) can cause an identical syndrome that is monospot negative. CMV may be associated with pharyngitis more commonly in children than in adults. An acute mononucleosis-like syndrome with pharyngitis may also be the presenting manifestation of primary HIV infection. Viruses in the hemorrhagic fever group produce an acute pharyngitis that occurs early in the disease, before skin lesions appear. Also, exudative pharyngitis is a common clinical manifestation in Lassa fever.
The differential diagnosis of acute pharyngitis generally centers upon the differentiation of streptococcal from viral etiologies. Features suggestive of streptococcal pharyngitis include tonsillar swelling, moderate to severe tenderness on palpation, enlargement of lymph nodes, presence of scarlatiniform rash, and absence of coryza. The presence of nasal symptoms or of conjunctivitis favors a viral etiology, and as described, some viral syndromes may present with distinguishing characteristics that help in their identification. Generally, acute pharyngitis in children less than 3 years of age is predominantly viral in origin. The presence of exudate is suggestive of bacterial etiology, but exudates may also be seen with adenovirus or EBV.
 PATHOGENESIS
PATHOGENESIS
As described, pharyngitis in the common cold is probably the result of chemical mediators of inflammation, which are potent stimulators of pain nerve endings. Potentially similar mechanisms may account for pharyngitis in other viral syndromes as well. Direct viral damage and other host inflammatory responses may also contribute. Pharyngitis occurs most often as part of the common cold syndrome and thus is usually associated with the same viruses that cause colds. In some cases, pharyngeal symptoms predominate to a degree that overshadows other complaints. The kinins are potent stimulators of pain nerve endings, and high levels of bradykinin and lysyl bradykinin are present in nasal secretions of patients with rhinovirus colds. Intranasal application of bradykinin promotes sore throat and nasal symptoms in volunteers, supporting a role for these agents in the pathogenesis of cold symptoms.
 DIAGNOSTIC TESTS
DIAGNOSTIC TESTS
Identification of viral causes of pharyngitis is generally possible through isolation in cell culture, but is seldom attempted in clinical practice. Rapid antigen detection tests are available for RSV and influenza A virus.
Rapid diagnostic tests are widely available for the office identification of group A streptococci, and are indicated in most cases in which the etiology is uncertain. When highly sensitive tests are used, backup cultures are generally not necessary. Routine studies for other bacterial and nonbacterial pathogens are usually not obtained. When guideline recommendations for the selective use of throat cultures are used with antibiotic treatment based only on positive rapid test or throat culture, results can reduce unnecessary use of antibiotics for treatment of pharyngitis.30
 TREATMENT AND PREVENTION
TREATMENT AND PREVENTION
The treatment of most cases of viral pharyngitis is symptomatic, as noted in the section on common colds. Patients suspected of having influenzal pharyngitis who are seen within the first 2 days of illness can be treated with antiviral therapy (see the discussion on influenza virus). In immunosuppressed patients with chronic herpetic pharyngitis or normal hosts with primary gingivostomatitis, acyclovir therapy is recommended (see the discussion on herpes simplex virus).
Treatment of group A streptococcal infections with antimicrobial agents is generally initiated to prevent rheumatologic complications of this infection, and because treatment of acute streptococcal pharyngitis is associated with more rapid resolution of symptoms,31 although the absolute benefits are rather modest.
CROUP
Important clinical features, aspects of pathogenesis, diagnosis, and management of croup are presented below.
 CLINICAL FEATURES
CLINICAL FEATURES
Croup, or viral laryngotracheobronchitis, is a clinically distinct illness that predominantly affects children under the age of 3. The illness typically begins with upper respiratory tract symptoms of rhinorrhea and sore throat, often with a mild cough. After 2 or 3 days, the cough deepens and develops a characteristic brassy, barking quality, which is similar to a seal’s bark. Fever between 38 and 40°C is common, although those with croup due to RSV may have normal temperatures. The child may appear apprehensive and most comfortable sitting forward in bed. The respiratory rate is elevated, but usually not over 50; this contrasts with bronchiolitis, in which more severe tachypnea is often seen. Chest wall retractions, particularly in the supraclavicular and suprasternal areas, may be observed. Children with this finding on presentation have a higher risk of hospitalization or of requiring ventilatory support.
The characteristic physical finding of croup is inspiratory stridor. Inspiration is prolonged, and in very severe cases, some degree of expiratory obstruction may also be seen. Rales, rhonchi, and wheezing, which reflect the characteristic involvement of the lower respiratory tract, may be heard on physical examination. A fluctuating course is typical for viral croup, and the child may appear to worsen or improve within an hour. The typical duration of croup is 3 to 4 days.
Hypoxemia occurs in 80% of children with croup severe enough to require hospitalization. The degree of hypoxia is generally difficult to ascertain clinically, but pulse oximetry provides a reliable and noninvasive means to monitor the state of oxygenation. Children who develop respiratory insufficiency as a result of increasing fatigue also may have elevations in PaCO2.
Children with croup characteristically exhibit subglottic narrowing of the tracheal air shadow on PA films of the neck, the so-called “steeple” sign). This finding may be useful in differentiating croup from epiglottitis. However, radiographs are limited in accuracy, and when the diagnosis is uncertain, radiologic and pharyngeal examination should be avoided because of the risk of cardiorespiratory arrest in acute epiglottitis. Emergency assessment by an otolaryngologist or an anesthesiologist is indicated in this situation. Chest X-rays may reveal parenchymal infiltrates which are part of the characteristic involvement of the lower respiratory tract in this syndrome.
 VIRAL ETIOLOGIES AND DIFFERENTIAL DIAGNOSIS
VIRAL ETIOLOGIES AND DIFFERENTIAL DIAGNOSIS
PIV-1 and PIV-2 are the most common viruses responsible for croup, accounting for about 75% of cases,32 and the seasonal incidence of croup reflects the seasonal variations in PIV incidence. Less common causes of croup include RSV, influenza A or B viruses, measles, rhinoviruses, and adenoviruses as well as Mycoplasma pneumoniae. PIV-2, influenza A viruses and measles are associated with more severe disease, but generally, the clinical presentation of the croup syndrome due to individual agents is similar.
The majority of cases of inspiratory stridor in children are caused by viral croup. However, it is critical to distinguish these syndromes from other, potentially more serious causes of airway obstruction such as bacterial epiglottitis and tracheitis early in clinical management. Epiglottitis is an acute cellulitis of the epiglottis and surrounding structures. Patients present with acute respiratory distress and drooling, but the barking cough of croup is absent. Since the introduction of effective vaccines for the major bacterial cause or epiglottitis, Haemophilus influenzae type b (Hib), the incidence of epiglottitis in children has also declined considerably. In adults, and rarely in children, epiglottitis may be caused by a variety of other bacterial agents such as Haemophilus parainfluenzae or alpha hemolytic streptococci, which may spread from a contiguous focus of infection. Bacterial tracheitis is a relatively rare syndrome that mimics croup. Abundant purulent sputum is often present. Bacterial tracheitis is usually caused by Staphylococcus aureus or Hib; other bacteria such as Streptococcus pneumoniae have also been associated with this syndrome. Other infectious causes of stridor, including peritonsillar or retropharyngeal abscess, or diphtheria, should be considered. Evidence of noninfectious causes of stridor such as trauma or aspiration of a foreign body, should also be sought in the history and physical examination.
 PATHOGENESIS
PATHOGENESIS
The severity of clinical symptoms in PIV croup appears to be directly related to the level of virus replication.33 The viral infection in croup produces inflammation both in the upper respiratory tract, and in the lung parenchyma. The classic signs of croup, including the barking cough and inspiratory stridor, arise mostly from inflammation occurring in the larynx and trachea. Inflammatory changes are seen by histology in the epithelial mucosa and submucosa of the larynx and trachea. The cellular infiltrate includes histiocytes, lymphocytes, plasma cells, and polymorphonuclear leukocytes. The inflammation and obstruction are greatest at the subglottic level, which is the least distensible part of the airway because it is encircled by the cricoid cartilage. Consequently, localized inflammation and edema lead to obstruction to airflow. The impeded flow of air through this narrowed area produces the classic high-pitched vibration. Obstruction is greater during inspiration because it occurs in the extrathoracic portion of the airway, and is enhanced in small children because the walls of the airways in these individuals are relatively compliant. Obstruction of airflow results in an initial decline in tidal volume, which is compensated by an increase in respiratory rate to maintain adequate alveolar ventilation. However, if the obstruction increases, the work of breathing may increase until the child tires, and as the respiratory rate declines, the child develops hypercarbia and respiratory failure.
Involvement of the lower respiratory tract with resulting hypoxia is integral to the pathophysiology of croup.34 Inflammatory changes are noted throughout the respiratory tract, including the linings of the bronchi, bronchioles, and even the alveoli. Although some degree of hypoxia can be explained on the basis of hypercarbia, the major pathophysiologic mechanism is ventilation–perfusion mismatching.
Pulmonary edema may complicate severe croup and upper airway obstruction. The onset of pulmonary edema often is immediately following intubation. Pulmonary edema in these cases does not appear to be due to pulmonary artery hypertension, but to local hypoxia, and increased alveolar–capillary transmural pressure.
In addition to these anatomic pathophysiologic events, immunologic mechanisms may also play a role in some manifestations or in determining the severity of disease. Virus-specific IgE responses appear earlier and are of greater magnitude in patients with PIV-associated croup than in age-matched controls with simple upper respiratory illness. Histamine is also detectable in upper respiratory tract secretions in this condition. There also appears to be a relationship between croup and subsequent reactive airways disease and/or heightened responsiveness to bronchodilators, particularly in children with recurrent croup.
 DIAGNOSTIC TESTS
DIAGNOSTIC TESTS
PIVs and other viruses associated with croup can be isolated in cell culture, and specific viral diagnosis can be made by PCR, but the clinical syndrome is sufficient for the diagnosis, and management generally does not depend on identification of the specific agent.
 TREATMENT AND PREVENTION
TREATMENT AND PREVENTION
Because the majority of hospitalized children are hypoxic, oxygen is the mainstay of treatment for severe disease, and should be given to all hypoxemic patients. Use of helium as the carrying gas, rather than air, has been suggested as a method to decrease the work of breathing. However, this approach requires use of 70% helium to be effective and limits the amount of oxygen which can be delivered.35 Humidified air, or mist therapy is commonly used, and has several potential roles. Desiccation of the inflamed epithelial surfaces is decreased, and the viscosity of the exudate is reduced. However, the value of mist therapy has not been proven, and it should be recognized that water from the standard home-use vaporizer cannot reach the lower respiratory tract because of the large particle size. In addition, removal of the child from the parents and placement in a mist tent can be more distressing to the child than beneficial.
Administration of nebulized racemic epinephrine generally gives rapid, symptomatic relief in croup.36 It is believed that alpha-adrenergic stimulation by this drug causes mucosal vasoconstriction, leading to decreased subglottic edema. Several randomized trials have demonstrated a rapid beneficial effect on airway obstruction.37,38 The onset of action is rapid, often within minutes, but the duration of relief is also limited, lasting 2 hours or less. Therefore, treated subjects should be observed closely for clinical deterioration. Although symptomatic relief is considerable, use of epinephrine is not associated with improvements in oxygenation, probably because the defect in oxygen is associated with ventilation–perfusion mismatching due to lower respiratory tract involvement. In addition, tachycardia may occur. Thus, inhaled epinephrine is generally reserved for children who fail to respond to more conservative management,39 although some centers use it routinely.
Steroids have been shown to confer significant benefits in the management of mild, moderate, and severe croup, including more rapid improvement in symptoms, reduced length of hospital stay, and reduced rates of intubation. Administration of intramuscular, oral, or nebulized steroids appears to be equally effective.40,41 Administration of single-dose steroid therapy in this setting has not been associated with significant side effects,42 and should probably be used in any patient with illness significant enough to require an emergency room or clinic visit.
Antiviral agents effective against some of the viruses responsible for croup are available, but have not been tested for efficacy in this situation. However, the potential benefit of the use of antiviral agents in the typical self-limited course of croup would likely be limited. Since croup is a viral illness, antibiotic therapy is of no benefit.
Effective prevention of croup will largely depend on the development of vaccines for the individual viral agents responsible for this syndrome. Vaccines are currently available for both measles and influenza. There are currently no vaccines available for PIV.
TRACHEOBRONCHITIS
Clinical features, pathogenesis, diagnosis, and management of tracheobronchitis are discussed below.
 CLINICAL FEATURES
CLINICAL FEATURES
In addition to causing croup and bronchiolitis, viral infection of the trachea and bronchi may cause tracheitis or tracheobronchitis. The diagnosis of acute bronchitis is usually applied to cases of acute respiratory disease with severe and prolonged cough that continues after other signs and symptoms of the acute infection have subsided. Cough occurs during the first week of illness in 30% of rhinovirus colds in young adults and in 80% or more of cases of influenza A virus infection, in which it is often prolonged. Adenovirus infections characteristically involve the tracheobronchial tree, with resultant bronchitis that in military populations is part of the syndrome of acute respiratory disease. Tracheitis is characterized by tracheal tenderness, which can be elicited by gentle pressure on the anterior trachea just below the cricoid cartilage. Substernal discomfort on inhalation and nonproductive paroxysmal cough are noted. Paroxysmal nonproductive cough is also characteristic of tracheobronchitis, and is usually much more severe at night. Later in the course of illness, small amounts of clear or whitish sputum may be produced. Accompanying symptoms may include fever, headache, myalgias, malaise, and anorexia. After several days of coughing, chest wall or abdominal discomfort that is muscular in nature may be noted. Physical findings are generally nonspecific; examination of the chest may reveal no adventitious sounds, but more commonly diffuse rhonchi and occasional wheezing. Physical signs such as egophony, pleural friction rubs, or areas of dullness to percussion, are not present.
 PATHOGENESIS
PATHOGENESIS
The mechanisms of cough production in viral infection are not well understood but may include direct damage to the respiratory mucosa, release of inflammatory substances in response to the infection, increased production and/or decreased clearance of respiratory secretions, and stimulation of airway irritant receptors. Intranasal application of several prostaglandins also produces cough in uninfected volunteers.43 Infection may also enhance airway reactivity, leading to increased sensitivity to cold air and pollutants such as smoke.
 VIRAL ETIOLOGIES AND DIFFERENTIAL DIAGNOSIS
VIRAL ETIOLOGIES AND DIFFERENTIAL DIAGNOSIS
Tracheobronchitis is most typically caused by influenza A or B virus. In adults other common respiratory viruses such as PIV or RSV may present with prolonged cough. Herpes simplex has been associated with necrotizing tracheobronchitis in nonimmunocompromised hosts. This syndrome is often accompanied by refractory bronchospasm.
The differential diagnosis of acute bronchitis includes nonviral infections and noninfectious etiologies such as cough-variant asthma. M. pneumoniae and Chlamydia pneumoniae infections cause prolonged cough. Bordetella pertussis infection should also be considered in the differential diagnosis. In otherwise healthy persons, workup of acute cough should be directed toward determining the presence of pneumonia.
 DIAGNOSTIC TESTS
DIAGNOSTIC TESTS
Influenza viruses can be isolated in cell culture, as can many of the other viruses associated with bronchitis. In addition, rapid antigen tests or nucleic acid tests are available for some of these viruses. Specific viral diagnosis is generally not necessary for appropriate management of bronchitis.
 TREATMENT AND PREVENTION
TREATMENT AND PREVENTION
Treatment in adults is best effected by prescribing rest, aspirin for headache and fever, cold water vapor inhalation; and a cough syrup such as guaifenesin with dextromethorphan. For children, a cough suppressant may be helpful. In the absence of signs of pneumonia, treatment of prolonged cough with antibacterial agents is of no benefit.44,45
BRONCHIOLITIS
Clinical features, pathogenesis, diagnosis, and management of bronchiolitis are discussed in this section. The reader is also referred to Chapter 51.
 CLINICAL FEATURES
CLINICAL FEATURES
Bronchiolitis is a fairly characteristic syndrome whose presenting symptoms are dominated by the major pathophysiologic defect, obstruction to expiratory air flow.46 The onset of lower respiratory symptoms is usually preceded by rhinitis, often with nasal congestion and discharge. More severe symptoms characteristically occur 2 to 3 days later, but in some cases are concurrent with the onset of upper respiratory symptoms. In many instances, there may be a history of exposure to an adult or sibling with a cold or other minor respiratory illness, or history of exposure to other cases of bronchiolitis in the day care setting.
The hallmark of disease is wheezing, which can be quite marked, with flaring of the nostrils and use of accessory muscles of respiration. Cough may or may not be prominent initially, and when cough is present, it may be paroxysmal in nature. Slight cyanosis is often observed, but the presence or absence of cyanosis is not a reliable indicator of the degree of oxygenation or of the severity of disease. Physical findings are generally confined to the chest, with the development of rales, which are usually musical in the beginning, and then become moister. Hyperresonance of the chest may be observed, and the liver may be displaced downward. The respiratory rate is elevated, with rates of from 50 to 80 breaths per minute. Fever is frequently present at the beginning of the illness, but may no longer be present at the time lower respiratory tract involvement develops. Among hospitalized infants, one-third or more are afebrile, but have marked lower respiratory tract disease. Thus, the presence or absence of fever does not indicate the severity of the child’s illness. Mild conjunctivitis is noted in about one-third of cases, with pharyngitis of varied severity in about one-half, and otitis media in 5% to 10%. The hospital course is variable, but most infants show improvement in 3 to 4 days.47
Radiologic findings are generally nonspecific, with reported findings including air trapping, consolidation, and collapse.48 However, there is no correlation between the chest X-ray findings and the clinical course.49 Chest X-rays should be obtained to rule out alveolar-filling defects suggestive of bacterial pneumonia and in those infants with severe disease, sudden deterioration, or underlying disorders. Results of routine laboratory tests are generally unremarkable. The peripheral white blood cell count is usually not elevated. Electrolyte disturbances, most notably, hyponatremia, may be seen with severe disease, particularly if excessive amounts of hypotonic fluid are administered. Acute disease may be associated with elevations in pulmonary artery pressure, but echocardiographic studies are usually unremarkable in infants with structurally normal hearts.
Bronchiolitis is a disease predominantly of infancy, and the peak age incidence is between 2 and 6 months of age, with over 80% of cases occurring in the first year of life. The risk of hospitalization and severe bronchiolitis is particularly high in infants with congenital heart disease, chronic lung disease, or immunodeficiency. In addition, infants born prematurely, and those who are less than 6 weeks of age at the time of presentation are also at increased risk of hospitalization. More severe disease has also been documented in children with a family history of asthma, and those exposed to cigarette smoke in the family setting.
 VIRAL ETIOLOGIES AND DIFFERENTIAL DIAGNOSIS
VIRAL ETIOLOGIES AND DIFFERENTIAL DIAGNOSIS
RSV causes the majority of cases of bronchiolitis and during the RSV epidemic season, essentially all cases are due to this virus. Overall, RSV is recovered from about three-fourths of all infants admitted to the hospital with bronchiolitis.46 Several other respiratory viruses are associated with bronchiolitis, including PIVs, influenza virus, mumps, and rhinoviruses. The epidemiology and pathophysiology of parainfluenza bronchiolitis are similar to that of RSV, and particularly severe disease may be associated with PIV-3. Adenoviruses types 3, 7, and 21 are relatively uncommon causes, but may be associated with more severe disease, including the development of bronchiolitis obliterans. Rhinoviruses represent a small but significant proportion of cases of bronchiolitis,50 and may mimic RSV infection in infants with bronchopulmonary dysplasia. Rhinoviruses and Mycoplasma pneumoniae become more important causes of infection-induced wheezing as children become older. Surveys of bronchiolitis in various parts of the world have suggested a similar pattern of viral etiologies.
A significant number of cases are associated with the human metapneumovirus (hMPV).51 The clinical picture most closely resembles that of RSV, and bronchiolitis is the major manifestation in children. Clinical features include wheezing and hypoxia. There are no clinical features that can distinguish between disease caused by hMPV and RSV, although generally those due to RSV may be more severe. Human coronaviruses have also been associated with lower respiratory tract disease in infants.52 An additional recently described human parvovirus, the human Boca virus, has been found in as many as 20% of cases of acute wheezing in young children. This virus is often detected in the presence of other viruses, and the exact role it plays in this syndrome has not been determined completely.53
The differential diagnosis of diseases characterized by expiratory airflow obstruction in infants is relatively small. Pertussis can occasionally be confused with bronchiolitis; however, more frequent vomiting and more paroxysmal cough would be clues to the diagnosis. Anatomic defects such as vascular rings can cause obstruction of the airway. Foreign bodies should be considered strongly, especially in young infants. Gastroesophageal reflux is an additional consideration. The major differential diagnostic consideration is asthma, which is uncommon under the age of 1 year.
 PATHOGENESIS
PATHOGENESIS
The pathophysiology of infectious bronchiolitis has been described most completely in the case of infection with RSV.46 The average incubation period is 4 to 5 days, with a range of 2 to 8 days. Virus replication is limited to the respiratory tract mucosa, which may be involved through its entire length. Involvement of the lower respiratory tract probably occurs by cell-to-cell spread through the respiratory epithelium or aspiration of upper respiratory secretions. Pathologic findings in RSV bronchiolitis include necrosis of bronchiolar epithelium, loss of ciliated epithelial cells, and marked peribronchiolar mononuclear inflammation. Virus-induced cytopathology and associated submucosal edema leads to obstruction of smaller bronchioles, particularly in infants, with distal collapse or air-trapping.
Viral infection of epithelial cells of the bronchioles leads to destruction and necrosis of the ciliated epithelium. Lymphocytes can be seen in increased numbers in the peribronchial tissues. The submucosa becomes edematous, and there is increased production of mucus. Ultimately, dense plugs of alveolar debris and strands of fibrin form within small bronchi and bronchioles, which may partially or completely obstruct airflow. The pathogenic basis for respiratory difficulty in bronchiolitis is related to obstruction of these small airways. Hypoxemia is the major abnormality of gas exchange, with ventilation–perfusion imbalance the major cause of the hypoxemia. In addition to hypoxia, hypercarbia and respiratory acidosis have been observed in some severely ill infants.
Infants are particularly susceptible to the consequences of viral infection for several reasons. The peripheral airways are disproportionately narrow in the early years of life. In addition, collateral channels of ventilation, such as the pores of Kohn, are deficient both in number and size in the infant lung. Finally, the airways of infants are intrinsically more reactive to bronchospastic stimuli than are the airways of older children.54
The possibility that immune responses are involved in the pathogenesis of RSV bronchiolitis has received considerable attention. The presence of pre-existing infection-induced antibody does not appear to play a role in enhancing the severity of disease because maternal antibody, or passively transferred antibody is protective. It has also been postulated that cellular immune responses may be involved in the pathogenesis, since infants infected with RSV who have clinical bronchiolitis have higher levels of cell-mediated immunity to RSV than those with uncomplicated upper respiratory tract disease. Immediate hypersensitivity type reactions have also been postulated to play a role in the pathogenesis of wheezing in bronchiolitis. Production of virus-specific IgE and subsequent release of mediators of bronchoconstriction have been documented with RSV and PIV bronchiolitis. Leukotriene C4, a potent stimulant of airway smooth muscle constriction and mucus secretion, is also released into the airway in acute bronchiolitis.55 Elevated levels of histamine and prostaglandin F2α metabolite have been found during acute bronchiolitis and increased levels of eosinophilic cationic protein in nasopharyngeal secretions, suggesting that release of chemical mediators of inflammation in response to viral antigens is one factor contributing to the development of severe bronchiolitis.
The innate immune response also plays an important role in the pathogenesis of RSV disease in infants, and it has been recognized that single nucleotide polymorphisms in several genes that control the inflammatory response have an important impact on the severity of RSV disease. Examples include polymorphisms in the genes for IL-4, IL-8, and IL-13, and in TLR-4 and the CCR5 receptor, among others (see Chapter 121).
Following recovery from acute bronchiolitis, some children experience continued episodes of wheezing, especially during viral upper respiratory infections. Estimates are that from 5% to 50% of children diagnosed as having bronchiolitis in infancy go on to develop recurrent episodes of wheezing. Generally wheezing episodes wane in frequency over the next several years. After clinical bronchiolitis, some individuals also may demonstrate increased bronchial responsiveness to histamine or cold challenge, which generally decreases over time. The mechanism by which RSV or other viral infection might lead to increased episodes of wheezing is unclear. Some individuals develop elevated levels of viral-specific IgE, which may play a role in triggering bronchospasm upon re-exposure. In some studies, persistent wheezing correlates with a family history of asthma, and with higher levels of IgE.
 DIAGNOSTIC TESTS
DIAGNOSTIC TESTS
Rapid viral diagnosis can be made by identification of viral antigens in nasopharyngeal secretions. Both immune-based assays, such as immunofluorescence or enzyme-linked immunosorbent assay (ELISA), and nucleic acid–based techniques, such as hybridization or polymerase chain reaction (PCR), have been developed. Immune-based techniques are generally preferable for routine diagnostic purposes, and several kits are commercially available. The sensitivity of such techniques is dependent on the quality of the nasopharyngeal specimen, with nasopharyngeal aspirates superior to brushings or swabs. Commercially available immunofluorescence or ELISA antigen detection has a sensitivity of about 75% to 90% relative to culture for specimens collected from children, who shed large quantities of virus. The sensitivity of such tests in adults, who shed lower quantities of virus, is much lower (generally less than 20%). In transplant patients with suspected RSV pneumonia, samples of the lower respiratory tract by bronchoalveolar lavage are more sensitive than throat swabs for the detection of RSV.
 TREATMENT AND PREVENTION
TREATMENT AND PREVENTION
Recommendations regarding the treatment and prophylaxis of bronchiolitis have been summarized recently.56 Correction of hypoxemia is the most important aspect of managing RSV lower respiratory tract disease. Oxygenation should be monitored by pulse oximetry, and oxygen administered to infants whose oxygen saturation consistently falls below 90%. Since bronchiolitis is a viral disease which is infrequently complicated by bacterial superinfection, routine treatment with antibiotics is not warranted. Because of the dehydrating effect of tachypnea and reduced oral intake in some hospitalized infants, parenteral rehydration is often needed, but care must be taken to avoid inducing hyponatremia. Fluid intake and electrolyte concentrations should be carefully monitored in all infants with severe bronchiolitis, as hyponatremia and syndrome of inappropriate secretion of antidiuretic hormone (SIADH) may occur.
Data concerning the potential benefits of bronchodilator therapy are somewhat conflicting. Generally, bronchodilators produce modest short-term improvements in clinical scores, but do not improve oxygenation, rates of hospitalization, or duration of hospital stay.57–59 In addition, bronchodilating drugs may contribute to increased restlessness and cardiovascular stress. Because some children do respond to these drugs,60 a reasonable strategy is a short trial of nebulized bronchodilator with continued therapy only in selected children who respond.
The majority of studies of systemic corticosteroids have also failed to demonstrate a beneficial effect in acute bronchiolitis,61,62 and oral corticosteroids do not appear to have a beneficial effect in terms of long-term outcomes, either. Steroids may also be associated with evidence for delayed viral clearance. Although a recent meta-analysis suggested that systemic steroids have a small but statistically significant effect in decreasing length of stay,63 the clinical significance is minimal; therefore, corticosteroids should not be used routinely.
Antiviral therapy with ribavirin (1-β-D-ribofuranosyl-1,2,3-triazole-3-carboxamide) remains controversial. Ribavirin is a broad-spectrum antiviral agent with antiviral activity against multiple respiratory viruses including RSV in a variety of cell culture systems and animal models. It does not achieve suitable levels in respiratory secretions when administered systemically, so that therapy of bronchiolitis has used aerosolized drug. Although initial randomized placebo-controlled trials of ribavirin small–particle aerosol showed benefit in treatment of bronchiolitis, subsequent experience with use of the drug in clinical practice did not confirm this clinical benefit. These findings, and the expense of this drug, suggest that ribavirin should be considered only in selected infants and young children with severe illness or at high risk for serious RSV disease.
Bronchiolitis due to RSV can be prevented by passive transfer of antibody against this agent. A humanized neutralizing monoclonal antibody to the RSV F protein, palivizumab (Synagis) has significant protective efficacy in a population of infants with prematurity or bronchopulmonary dysplasia, as well as in children with hemodynamically significant congenital heart disease. Current recommendations for the use of passive antibody prophylaxis are to consider use in infants and children less than 2 years of age with chronic lung disease or congenital heart disease, and in infants born at 32 weeks of gestation or earlier (who would be expected to receive little placental transfer of maternal antibody). Palivizumab may be considered for infants born between 32 and 35 weeks who have at least two additional risk factors, such as exposure to second-hand smoke or attendance at day care.56 Palivizumab is not effective for therapy of RSV disease.
Interruption of nosocomial transmission may be facilitated by thorough handwashing, decontamination of surfaces and inanimate objects, and isolation or cohorting of infected infants. Use of disposable eye–nose goggles by pediatric staff reduces the risk of nosocomial RSV infection in both staff and patients. Regular use of gowns, gloves, and possibly masks by hospital staff caring for infected children may also reduce the risk of nosocomial RSV spread. Protective isolation of high-risk infants or deferring their elective admission has been recommended during institutional outbreaks of RSV.
Vaccines are available to prevent bronchiolitis due to influenza virus and mumps, but there is no vaccine currently available for prevention of bronchiolitis due to RSV. There are multiple significant hurdles to the development of such a vaccine, including the very young age at which the disease presents, the suppressive effect of maternal antibody on vaccine responses, and the potential for enhanced disease in vaccine recipients.64
INFLUENZA
Clinical features, epidemiology, pathogenesis, and diagnosis of influenza are discussed below. Treatment and prevention are discussed separately in the section that follows.
 CLINICAL FEATURES
CLINICAL FEATURES
The onset of influenza is typically abrupt, and the illness is characterized by the predominance of systemic symptoms, including fever, prostration, myalgias, and malaise. Respiratory symptoms may be relatively minimal, particularly early in the course, and include nasal complaints, sore throat, hoarseness, and nonproductive cough. Because of the involvement of tracheal epithelium in infection, complaints of burning throat and substernal pain may be seen. Among healthy adults, the best clinical predictors of influenza virus positivity are the presence of cough and fever in patients presenting during epidemics.65
Other than fever, there are usually few findings on physical examination. Affected individuals may exhibit rhinitis, pharyngitis, conjunctival injection, and tracheal tenderness. The chest is usually clear in uncomplicated cases. Most acute symptoms resolve in 3 to 5 days, but complete recovery may take weeks. The clinical features of influenza A and B virus infection are similar.
Influenza is an important cause of acute febrile illness in children during epidemics. Generally symptoms of influenza are similar to those in adults, although children may have higher fever with febrile seizures. As described, influenza may be associated with otitis media or croup in children.
Pneumonia represents the most severe complication of influenza virus infection in both normal and compromised hosts. Primary influenza viral pneumonia is characterized by rapid progression of dyspnea, cough and cyanosis, and the development of acute respiratory distress syndrome (ARDS). Chest roentgenographs reveal bilateral interstitial infiltrates, sputum production is scanty, and Gram stain reveals few organisms. Secondary bacterial pneumonia may present 1 to 2 weeks after apparent recovery from an acute influenza episode with recurrence of fever, and signs and symptoms of typical lobar pneumonia. Pneumonia is described in more detail below.
 EPIDEMIOLOGY
EPIDEMIOLOGY
Influenza is typically associated with seasonal epidemics of greater or lesser severity, which occur during the winter months in temperate climates in both the northern and southern hemispheres. Outbreaks in the northern hemisphere are usually noted between the months of November and March or April, and typically last for from 4 to 6 weeks in any given community. The reasons for the seasonal behavior of influenza are not known but have been the subject of much interest. Some suggested mechanisms have included the seasonal effects of heat and humidity on the viability of virus in the environment, behavioral patterns that facilitate transmission, such as attendance at schools, or sunlight effects on baseline immune function.
Seasonal influenza epidemics are regularly associated with excess morbidity and mortality,66 and both influenza A and B can be associated with severe illness. During interpandemic years, influenza is characterized by a “U-shaped” epidemic curve, in which attack rates and medically attended illness rates are generally highest in infants and young children, whereas mortality is generally highest in the elderly. Young children are generally more susceptible to influenza infection than are adults, and shed higher levels of virus for longer periods. Healthy children younger than 1 year of age are hospitalized for influenza at rates comparable to those of older children and adults with chronic high-risk conditions67,68 and are special targets of prevention measures.
Excess morbidity and mortality are particularly high in those with underlying medical conditions, including chronic cardiovascular or pulmonary disease, chronic metabolic disease including diabetes mellitus, renal dysfunction, hemoglobinopathies, or immunodeficiency, including HIV. Recently, it has been recognized that individuals with neuromuscular conditions affecting the ability to clear respiratory secretions,69 and persons with morbid obesity,70 are also at high risk for influenza hospitalization. Women in all stages of pregnancy, and in the immediate postpartum period, also have increased rates of hospitalization, ICU admission, and deaths during influenza epidemics.71,72
Antigenic variation is the hallmark of the epidemiology of influenza, and occurs in two forms referred to as antigenic “drift” and antigenic “shift.” Drift refers to the gradual accumulation of mutations, primarily in the hemagglutinin (HA) and neuraminidase (NA) proteins of the virus, that allow the virus to escape from antibody generated against previous strains. New, drifted strains generally appear every several years, and tend to replace previous strains. Antigenic drift occurs in both influenza A and influenza B viruses. In addition, recent influenza B viruses have evolved into two antigenically distinct lineages termed the “Yamagata” and “Victoria” lineages,73,74 which currently cocirculate.
Influenza A viruses exist as multiple antigenically distinct HA or NA subtypes. There are currently recognized 17 HA subtypes (H1–H17) and 9 NA subtypes (N1–N9), although current seasonal viruses are restricted to the H1N1 and H3N2 subtypes. When one influenza A subtype is replaced by another, a phenomenon recognized as antigenic shift, the new virus encounters a population with little or no prior immunity. World-wide epidemics of severe disease, referred to as pandemics, are usually the result.
Pandemics associated with antigenic shift have been noted multiple times in the 20th and 21st centuries. In 1918, H1N1 viruses emerged and replaced previous H3N2 viruses, in 1957 H2N2 viruses replaced H1N1 viruses, and in 1968 H3N2 viruses replaced the H2N2 viruses. In 1977, an H1N1 virus antigenically similar to H1N1 viruses from 1950 emerged to cause disease primarily in born after 1950, but did not replace previous H3N2 viruses. Finally, in 2009 a new H1N1 virus (the so-called pH1N1 virus or “swine flu”) emerged that was antigenically similar to H1N1 viruses prior to 1947 and cause disease predominantly in young adults and children. Since then, both H3N2 and pH1N1 viruses have circulated in humans. Influenza A viruses are zoonotic in multiple animal species including migratory waterfowl and swine, and epidemiologic data strongly support the notion that new pandemic influenza A viruses arise from this reservoir (see below).
 PATHOGENESIS
PATHOGENESIS
The mechanisms responsible for the transmission of influenza viruses from person to person have never been completely defined, and it is likely that both large particles (i.e., droplet) and small particles (aerosol) released from the airway during coughing and sneezing play a role. Once virus is deposited on the respiratory tract epithelium, it can attach to and penetrate columnar epithelial cells if not prevented from doing so by specific secretory antibody (IgA), by nonspecific mucoproteins to which virus may attach, or by the mechanical action of the mucociliary apparatus. The duration of the incubation period to the onset of illness and virus shedding varies from 18 to 72 hours depending in part on the inoculum dose. Viral shedding in the respiratory tract is first detected just before the onset of illness (within 24 hours), rapidly rises to a peak and remains elevated for 24 to 48 hours, and then rapidly decreases to low titers. Usually influenza virus is no longer detectable after 5 to 10 days of virus shedding. Both the duration as well as the level of virus shedding is typically longer in young children than in adults and in immunocompromised individuals. In general, the course of the clinical illness correlates temporally with the pattern of virus shedding, and severity is correlated with the quantities of virus shed.
Bronchoscopy of individuals with typical, uncomplicated acute influenza has revealed diffuse inflammation of the larynx, trachea, and bronchi, with mucosal injection and edema. Histologic findings on autopsy of more severe cases show extensive necrotizing tracheobronchitis, with ulceration and sloughing of the bronchial mucosa, extensive hemorrhage, hyaline membrane formation, and a paucity of polymorphonuclear cell infiltration. Abnormalities of pulmonary function are frequently demonstrated in otherwise healthy, nonasthmatic young adults with uncomplicated (nonpneumonic) acute influenza. Demonstrated defects include diminished forced flow rates, increased total pulmonary resistance, and decreased density-dependent forced flow rates consistent with generalized increased resistance in airways less than 2 mm in diameter,75 as well as increased responses to bronchoprovocation. In addition, abnormalities of carbon monoxide diffusing capacity and increases in the alveolar–arterial oxygen gradient have been seen. Of note, pulmonary function defects can persist for weeks after clinical recovery.
 VIRAL ETIOLOGIES AND DIFFERENTIAL DIAGNOSIS
VIRAL ETIOLOGIES AND DIFFERENTIAL DIAGNOSIS
Generally, clinical features alone cannot distinguish between the syndrome of influenza caused by either influenza A or B viruses, although attention to ongoing community surveillance can be very helpful in assessing the likely agents. Influenza-like illness can also be caused by a variety of other viruses, including RSV, adenovirus, and PIV. Knowledge of the currently circulating viruses can be very helpful.
 DIAGNOSTIC TESTS
DIAGNOSTIC TESTS
Rapid viral diagnosis based on immunologic detection of viral antigen in respiratory secretions is widely used for influenza diagnosis. All of these tests are designed to detect both influenza A and B, but only some of the tests differentiate between the two. Some of the tests that are minimally complex may be eligible for waivers that allow them to be used in the office setting. In general, sensitivities in adults and elderly patients tend to be lower than reported in young children, who tend to shed much larger quantities of virus in nasal secretions and therefore have much higher concentrations of antigen in their samples. Although all types of respiratory samples can be used in such tests, the sensitivity appears to be better with nasopharyngeal swabs and aspirates than with throat swabs or gargles.
Molecular diagnostic tests are becoming increasingly popular for influenza diagnosis, and are frequently coupled with the detection of other respiratory viruses, especially RSV. Nasopharyngeal samples remain the most sensitive of samples, but it has been noted that in some patients with pneumonia, sputum samples have been positive when nasal samples were negative. As is the case for all diagnostic testing, specific influenza testing should be focused on those cases where a specific diagnosis will have implications for therapy or infection prevention.76
TREATMENT AND PREVENTION
Treatment and prevention of influenza are considered with respect to vaccines, antiviral therapy, and antiviral prophylaxis.
 VACCINES FOR INFLUENZA
VACCINES FOR INFLUENZA
Two types of vaccines, inactivated and live, are currently available for the prevention of influenza (Table 130-3). Inactivated influenza vaccines are designed to induce primarily serum antibody to the HA and NA. Multiple formats of inactivated vaccines are available, including inactivated, chemically disrupted and purified HA and NA antigens generated from viruses grown in embryonated hen’s eggs or from viruses grown in cell culture, and purified HA recombinant protein expressed in insect cells using baculovirus technology. Virion-based vaccines are typically formulated to contain 15 μg of each HA in the final product, while the recombinant vaccine contains 45 μg, and a recent, high-dose vaccine containing 60 μg of each HA is licensed for use in individuals 65 and older. Current vaccines contain one example of influenza A (H1N1), and A (H3N2), based on the most current epidemiologic predictions of the likely circulating strains in the coming season, and may contain one example of influenza B (trivalent vaccine, or IIV3) or examples of both influenza B lineages (quadrivalent vaccine, or IIV4). Current recommendations do not include a preference for any specific format of inactivated vaccine, although this may change as more data become available.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


