CHAPTER
10
Ventricular Tachycardia
UNDERSTANDING AND MANAGING VENTRICULAR TACHYCARDIA (VT)
General Information
○Tachyarrhythmia of ventricular origin (originates distal to the bifurcation of the bundle of His) at a rate >120 bpm
○ Non-sustained: ≥3–5 beats in duration but self-terminates within 30 seconds.
○ Sustained: ≥30 seconds or requires termination due to hemodynamic instability within 30 seconds.
○ Complex ventricular ectopy: >10 premature ventricular contractions (PVCs)/hour, couplets, triplets, or non-sustained VT
▪ Complex ventricular ectopy confers an increased risk of death if found in association with a structurally abnormal heart; there is no increased risk for a normal heart.
Epidemiology and Clinical Features
○Tolerability depends on the rate, cardiac function, and peripheral compensation.
○ Asymptomatic (with or without electrocardiogram (ECG) changes)
▪ Usually due to a slower VT (rate <200 bpm)
○Potential symptoms attributable to ventricular arrhythmias include:
▪ Palpitations: Usually paroxysmal
▪ Presyncope: Dizziness, light-headedness, feeling faint, “greying out”
▪ Syncope: A sudden loss of consciousness with loss of postural tone with spontaneous recovery may be associated with myoclonic jerks mimicking seizure.
▪ Chest pain, dyspnea, and/or fatigue are usually related to underlying heart disease.
○Sudden cardiac death
Anatomy and Physiology (Mechanism)
○Pathophysiologic mechanisms of VT (see Table 10.1)
Table 10.1 Mechanisms of Ventricular Tachycardia
| Reentry | Abnormal Automaticity | Triggered Activity | |
VT morphology | Monomorphic | Monomorphic or polymorphic | Monomorphic or polymorphic |
Onset/termination | Abrupt | “Warm-up/cool-down” | “Warm-up/cool-down” |
Inducible at EPS | Inducible • Programmed stimulation | Not inducible | Inducible • Initiated by adrenergic activation and rapid rates • Terminated by verapamil, diltiazem, and/or adenosine |
Etiology | Underlying heart disease with myocardial scarring (permanent substrate) or acute ischemia | Metabolic changes • Ischemia, hypoxemia • ↓ Mg, ↓ K • Acid-base disturbances | Pause-dependent • Phase 3 (early afterdepolarization [EAD]) Catecholamine-dependent • Phase 4 (delayed afterdepolarization [DAD]) |
Risk | Permanent substrate | Reversible substrate | Permanent (genetic or heart disease) or reversible (e.g., due to drug or electrolyte imbalance) substrate |
Classification
Monomorphic VT
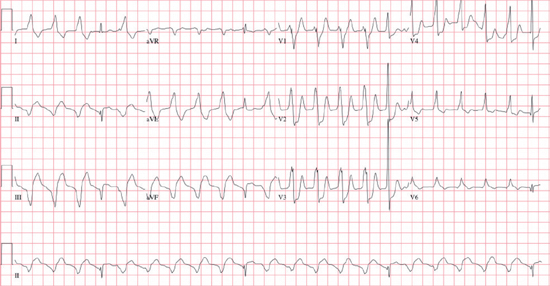
○Etiology and classification:
▪ Reentrant VT
• Scar-related: Slow conduction from myocardial fibrosis or scar
▫ Old myocardial infarction (MI)
▫ Dilated cardiomyopathy (DCM)
▫ Arrhythmogenic right ventricular cardiomyopathy (ARVC)
▫ Congenital heart disease with surgical scar (e.g., Tetralogy of Fallot)
• Reentry within the conduction system
▫ Fascicular VT (left posterior fascicular VT most common)
▫ Bundle branch reentry (ischemic cardiomyopathy or non-ischemic DCM with associated His-Purkinje disease)
▪ Enhanced automaticity
• Primary (idiopathic) VT
▫ Outflow tract VT (75%): Right ventricular outflow tract (RVOT)-VT, left ventricular outflow tract (LVOT)-VT, aortic cusp VT
▫ Non-outflow tract VT: Papillary muscle, mitral annular, tricuspid annular
▫ Acute post MI or surgery (myocardial injury)
▪ Triggered activity
• Acute post MI (usually arising near the His-Purkinje system)
Polymorphic VT
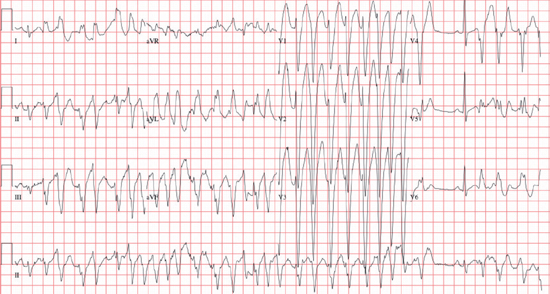
○Unstable VT with beat-to-beat QRS morphology variation (cycle length [CL] between 180 and 600 ms)
○Etiology and classification:
▪ Normal baseline QT
• Pathophysiology
▫ Can be due to reentry (e.g., acute MI, often degenerates to ventricular fibrillation [VF]), delayed afterdepolarization (e.g., catecholaminergic polymorphic VT (CPVT))
▫ Related to conditions of high sympathetic tone
▫ Acute ischemia (multiple reentrant circuits; abnormal automaticity)
▫ Channelopathies: Catecholaminergic polymorphic VT, Brugada syndrome, idiopathic polymorphic VT (PMVT)/VF
▪ Prolonged baseline QT
• Torsades de pointes
▫ Defined as a PMVT with a QRS amplitude and cardiac axis rotation over a sequence of 5–20 beats.
▫ Usually it is not sustained but recurs if the underlying cause is not corrected.
• Pathophysiology
▫ Due to early afterdepolarization
▫ Typical variant: Initiated by “short-long-short” coupling intervals (pause-dependent, typical for drug-induced)
▫ Short coupled variant: Initiated by “normal-short” coupling (induced by stress or startle, typical for congenital syndromes, adrenergic dependent)
• Etiology
▫ Acquired prolonged QT: Drugs (class Ia, III antiarrhythmic drug [AAD], phenothiazines, tricyclic antidepressant [TCA]), low Mg, or K
▫ Congenital prolonged QT
▪ Short QT syndrome
Ventricular Fibrillation (VF)
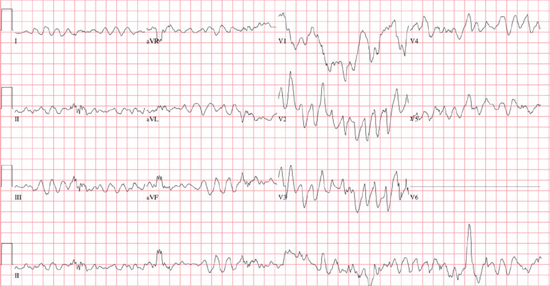
○Chaotic, rapid, disorganized wide-complex tachyarrhythmia (>300 bpm)
○Thought to be due to multiple reentrant wavefronts within the ventricle (wavelet hypothesis).
○All VT may degrade to VF.
○Etiology of primary VF is similar to polymorphic VT.
12-Lead ECG
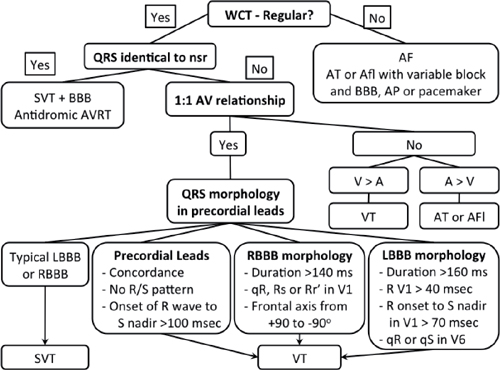
WCT: wide-complex tachycardia; SVT: supraventricular tachycardia; AVRT: atrioventricular reciprocating tachycardia; LBBB: left bundle branch block; RBBB: right bundle branch block.
○Ventricular rate 120–300 bpm (usually around 170)
○P waves
▪ AV dissociation (complete AV block) or retrograde P waves (intact VA conduction)
○Axis
▪ Change in axis of >40° from baseline or extreme (right superior) axis deviation
○QRS morphology and duration
▪ Left bundle branch morphology (Negative QRS complex in V1 – QS, rS) >160 ms
▪ Right bundle branch morphology (Positive QRS complex in V1 – qR, R, Rs, RSR′) >140 ms
▪ Variable with relatively narrow complexes in fascicular tachycardia (110–140 ms)
• Right bundle with left axis: Left anterior fascicular tachycardia
• Right bundle with right axis: Left posterior fascicular tachycardia
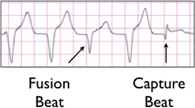
○ Fusion beat (almost pathognomonic of VT; generally only occurs with rates <160 bpm)
▪ A hybrid QRS is a result of the combination of normal atrial conduction down the His-Purkinje system and cell-to-cell conduction of a ventricular impulse.
▪ Fusion beats can also occur with PVC, ventricular escape, accelerated idioventricular rhythm, and Wolff-Parkinson-White syndrome (WPW).
○ Capture beat (pathognomonic of VT; generally only occurs with rates <160 bpm)
▪ Atrial impulse induces ventricular activation via the normal conduction system, resulting in a normal narrow QRS that is earlier than expected in the cardiac cycle.
○See page 91 about the differentiation of VT from SVT
○When describing VT, it is important to comment on:
▪ Duration: Non-sustained or sustained (lasts >30 seconds or associated with hemodynamic instability)
▪ Variability: Monomorphic or polymorphic
▪ Ventricular rate
▪ QRS morphology: Left or right bundle branch block morphology
▪ Axis: Right/inferior or left/superior
Localizing the Exit Site
○QRS morphology
▪ Left bundle morphology (negative QRS complex in V1: QS, rS): RV or LV septum
▪ Right bundle morphology (positive QRS complex in V1: qR, R, Rs, RSR′): LV
○Axis
▪ Superior (negative in II, III, aVF): Inferior wall or inferior septum
▪ Inferior (positive in II, III, aVF): Anterior wall or anterior septum
▪ Rightward: Lateral LV wall or apex
○Precordial transition
▪ Left bundle morphology VT
• ≤V3: Basal LV; RV septum
• ≥V4: Apical LV; RV free wall
• Negative concordance (all negative QRS V1–V6): Apical LV
▪ Right bundle morphology VT (reverse transition)
• ≤V2: Basal LV
• V3–V4: Mid cavity LV
• ≥V5: Apical LV
• Positive concordance (all positive QRS V1–V6): Mitral valve apparatus
○Variants and other features:
▪ Outflow tract VT (~LBBB morphology with an inferior axis)
• V1, V2 R-wave duration
▫ >50% of QRS: LVOT (left coronary cusp)
▫ <50% of QRS: RVOT or LVOT (right coronary cusp)
• V2 R:S ratio
▫ >1: left ventricular outflow tract (LVOT)
▫ <1: right ventricular outflow tract (RVOT)
• QRS transition in tachycardia compared to normal sinus rhythm (NSR)
▫ Earlier transition in NSR: RVOT
▫ Earlier transition in PVC/VT: LVOT
• Localization within the RVOT (see Table 10.2)
Table 10.2 12-Lead ECG Characteristics of VT with a RVOT Focus
| Anterior | Posterior | Middle | Septum | RV Free Wall | |
Precordial R/S transition | — | — | — | ≤V3 | ≥V4 |
Lead I and aVL | Neg. (qs or rS) | Pos. (R or Rs) | Pos. (rs or qrs) | Neg. | Pos. |
II, III, aVF | — | — | — | Monophasic | Notching |
▪ Localization of LV Basal VT: RBBB morphology (see Table 10.3)
Table 10.3 12-Lead ECG Characteristics of VT with a LV Basal Origin and RBBB Morphology
| Lead I | Lead V1 | Precordial Transition | |
Septal/parahisian | R or Rs | QS or Qr | Early (≤V2) |
Aorto-mitral continuity | Rs or rs | qR | Positive concordance |
Superior mitral annulus | rs or rS | R or Rs | Positive concordance |
Superolateral mitral annulus | rS or QS | R or Rs | Positive concordance |
Lateral mitral annulus | rS or rs | R or Rs | Positive concordance |
Papillary muscle | No Q | qR or R |
▪ Epicardial VT
• QRS duration >198 ms
• The initial portion of the QRS is delayed.
▫ Pseudo-delta wave ≥34 ms (QRS onset to earliest sharp deflection in any lead)
▫ Intrinsicoid deflection in V2 ≥85 ms (QRS onset to earliest S wave nadir)
▫ RS complex duration >121 ms (QRS onset to earliest R wave peak in any lead)
▫ Maximum deflection index >55% (QRS onset to peak R or S wave nadir/QRS duration)
• Localizing the epicardial exit site
▫ QS in I or aVL: Anterolateral/lateral LV
▫ QS in II, III, aVF: Inferior (near the middle cardiac vein)
▫ Loss of R from V1 to V2 then prominent R in V3 (near the anterior interventricular vein)
Other Investigations
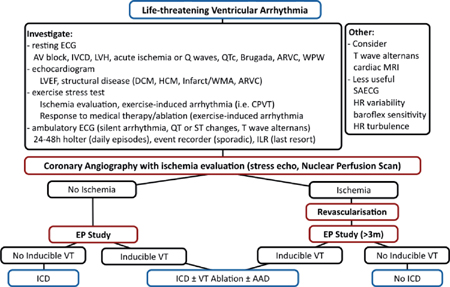
ARVC: arrhythmogenic RV cardiomyopathy; CPVT: catecholaminergic polymorphic ventricular tachycardia; DCM: dilated cardiomyopathy; HCM: hypertrophic cardiomyopathy; HR: heart rate; ILR: insertable looprecorder; IVCD: intraventricular conduction delay; LVEF: LV ejection fraction; LVH: LV hypertrophy; MRI: magnetic resonance imaging; SAECG: signal averaged ECG; WMA: wall motion abnormality; WPW: Wolff-Parkinson-White syndrome.
Management
Acute Management
○Non-sustained VT and PVCs
▪ No evidence that suppression of non-sustained VT (NSVT) prolongs life except with very rapid or repetitive (incessant) NSVT that compromises hemodynamic stability.
▪ First-line therapy
•β-blockers
▪ Second-line therapy
• Amiodarone + β-blockers
• Sotalol
• Catheter ablation
○Sustained monomorphic VT
▪ First-line therapy
• Direct-current cardioversion (DCCV)
▪ Second-line therapy
• IV procainamide is a reasonable alternative; use with caution if it is congestive heart failure (CHF) or hypotension.
• IV lidocaine: This is more effective if the cause is ischemic.
• IV amiodarone
▫ Hemodynamically unstable, refractory to cardioversion, or recurrent despite AAD
• Transvenous pace-termination
▫ Refractory to cardioversion, recurrent despite medical therapy
○Polymorphic VT with a normal baseline QTc
▪ First-line therapy
• DCCV
• IV β-blockers
• IV amiodarone
• Note: Calcium-channel blockers (CCB) may be effective for DAD (caused by inward calcium current).
• For Brugada or idiopathic VF consider IV isoproterenol infusion (target HR >100–120 bpm) or PO quinidine.
○Polymorphic VT with a prolonged baseline QTc
▪ Treat the underlying cause.
• Remove offending or unnecessary drugs (including AAD).
• Treat ischemia.
• Electrolyte abnormalities (keep K+ >4.0–4.5 mmol/L)
▪ Adjunctive therapy
• IV magnesium sulfate
▫ This is only useful with prolonged baseline QTc.
• Pacing
▫ Overdrive pacing (acute)
▫ Chronic pacing: Pause-dependent TdP
• Isoproterenol
▫ IV infusion to a target HR >100–120 bpm
• IV lidocaine or mexilitine
▫ LQT3 with TdP
Chronic Management
Table 10.4 Strategies for Chronic Management of Ventricular Arrhythmias
| Polymorphic VT/VF <48 h After Revascularization or Monomorphic VT in a Structurally Normal Heart | Polymorphic VT/VF >48 h After Revascularization or Monomorphic VT in a Structurally Abnormal Heart (LVEF <40%) | |
Risk of recurrence | Low | High (20%–30% mortality) |
ICD | No benefit (<40d post MI: CABG-PATCH, DINAMIT, IRIS) | Major benefit (CIDS, AVIS, CASH) |
Medical therapy | β-blocker (76% RRR) ± amiodarone (CASCADE, EMIAT/CAMIAT: ↓ VF/SCD but did not alter mortality post MI) Sotalol (use with caution in severe CHF or LV dysfunction) | β-blockers (first-line) If arrhythmia/shock • Add amiodarone (load then 200 mg/d: OPTIC) If resistant: • Increase amiodarone to 300–400 mg/d If still resistant: • Consider catheter ablation (or add mexilitine) If amiodarone side effects: • Dofetilide ± class I or β-blockers • Sotalol ± class I |
Other | Verapamil > Propafenone | Catheter ablation (see below) Surgical resection (e.g., LV aneurysm) or transplant |
ICD: implantable cardioverter-defibrillator; SCD: sudden cardiac death.
▪ An ICD may be the only way to reduce the risk of death in the majority of high-risk patients.
▪ Primary prevention
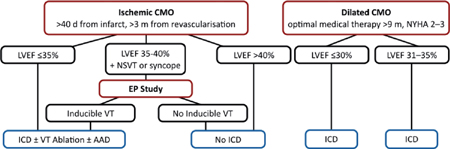
○Pharmacologic therapy
▪ Suppression of ventricular ectopy with AADs does not decrease the risk of SCD.
• CAST: Suppression of ambient ventricular ectopy either increased mortality (encainide, flecainide) or had no effect (moricizine).
▪ Empiric amiodarone may reduce the arrhythmia burden but it does not generally reduce the risk of SCD.
• CHF-STAT: 674 patients; LVEF <40%; complex ectopy
▫ No difference in survival overall vs. placebo (non-ischemic dilated cardiomyopathy [NIDCM] had a trend towards increased survival)
• CASCADE: 228 patients; survivors of VF arrest
▫ Amiodarone resulted in less ICD shocks or syncope vs. other AAD
• CAMIAT: 1202 patients; survivors of MI; complex ectopy
▫ No survival benefit vs. placebo
• EMIAT: 1500 patients; survivors of MI; LVEF <40%
▫ No survival benefit vs. placebo
• SCD-HeFT: 2521 patients; LVEF ≤35%; NYHA 2–3
▫ No survival benefit with amiodarone
○Invasive therapy
▪ Ablation of reentrant foci is an effective method of treating some types of VT.
CATHETER ABLATION OF VENTRICULAR TACHYCARDIA (VT)
Indications
○Frequent monomorphic PVCs and NSVT
▪ This is particularly true if it is associated with LV dysfunction (LV dilatation or decline in LVEF).
○Monomorphic VT: Sustained (class I), non-sustained (class IIa)
▪ Patients may be drug resistant or drug intolerant.
▪ They do not want a long-term drug therapy.
○Bundle-branch reentrant VT (class I)
○Adjunctive therapy in those with an ICD (class I)
▪ Patients receiving multiple shocks as a result of sustained VT that cannot be managed by device programming, changes in AAD therapy, or they do not want long-term drug therapy.
Anticipated Success
○Idiopathic ventricular tachycardia (e.g., RVOT or fascicular VT) 80%–90%, if inducible
○Bundle branch reentry: 80%–90%
○Ischemic VT: 60%–70%
○Dilated cardiomyopathy: 50% (usually requires epicardial ablation)
○ARVC: 70% (usually requires epicardial ablation)
Anticipated Complications
○Similar to all invasive ablation procedures
○3%–5% major complications
▪ Vascular access: hematoma, AV fistula, arterial pseudoaneurysm
▪ Catheter manipulation: vascular damage, microemboli/stroke, coronary dissection
▪ RF application: cardiac perforation/tamponade, coronary damage, AV block
○Mortality 1%–3%
Patient Preparation
○Use echocardiography (± contrast) to exclude the presence of LV thrombus (if LV ablation is anticipated).
○Stop all AAD for 5 half-lives before the procedure (especially for RVOT VT, fascicular VT).
○Conscious sedation is preferred to general anesthesia due to the risk of rendering the VT non-inducible.
Set-Up
○3D mapping system
▪ Focal VT: Set window to 50–80 ms prior to surface QRS onset
▪ Reentrant VT: Set window to >90% of tachycardia cycle length
○Diagnostic catheters
▪ Quadripolar catheters in right ventricular apex (RVa) and at the His
▪ Deflectable decapolar in CS (reference and pacing)
○Endocardial RV VT
▪ Non-irrigated RF: D-curve (medium/blue), C-curve (green), or bidirectional [D-curve (medium/blue)/F-curve (large/orange)]
▪ Irrigated RF: D-curve (medium/blue) or bidirectional [D-curve (medium/blue)/F-curve (large/orange)]
▪ Consider long sheath for improved stability in the RVOT (LAMP, SL0, or steerable sheath).
○Endocardial LV VT
▪ Irrigated RF ablation: F-curve (large/orange), D-curve (medium/blue), J-curve (extra large/black), or bidirectional (D/F, D/J, F/J)
▪
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


