Ventricular Septal Defects
Meryl S. Cohen
Leo Lopez
A ventricular septal defect (VSD) is a communication between the left and right ventricles or the left ventricle and right atrium. Other than bicuspid aortic valve, VSD is the most common type of congenital heart abnormality, accounting for up to 40% of congenital heart defects (1). VSDs encompass a wide anatomic and physiologic spectrum of disease, and they can occur as isolated defects or in association with other congenital heart lesions. Important features of VSDs include location, size, borders, and number of defects. Small defects are often hemodynamically insignificant except for a slightly higher risk of endocarditis (2). Large defects typically cause heart failure and require surgical intervention. Long-term outcome after surgical closure of VSD is generally excellent.
Epidemiology
It is difficult to determine the true incidence of VSDs because many close spontaneously in utero or in early infancy. The incidence ranges from 5 to 50 per 1,000 live births and 0.3 per 1,000 adults (3). Between 10,000 and 11,000 infants with an isolated VSD are diagnosed annually in the United States (4). Approximately one-fifth of these patients undergo surgical closure (5). VSDs are seen in association with many other congenital heart anomalies, including conotruncal defects such as tetralogy of Fallot, truncus arteriosus, double outlet ventricle, and aortic arch obstruction.
Etiology
The etiology of a VSD is likely multifactorial with both genetic and environmental components. VSDs are common in aneuploidy syndromes such as Trisomy 13, 18, and 21 (6,7,8) and in other genetic syndromes such as Holt–Oram (TBX5 mutation) (9). VSD in offspring has been associated with paternal use of marijuana and cocaine as well as paternal exposure to paint stripping (10,11).
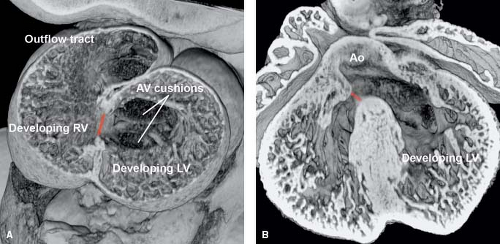
In the embryo, ventricular septation begins when the right ventricle expands after ventricular looping. The transitional zone between the embryonic left and right ventricles forms the primary interventricular foramen (12,13,14). This foramen separates the embryonic atrium and left ventricle from the right ventricle and outflow tract (Fig. 30.1A). Fusion of the superior cushions in the outflow tract forms the muscular subpulmonary and subaortic
outlets (conus) (15). The muscular portion of the ventricular septum is formed from the trabecular septum (between the embryonic left and right ventricles), the inlet component (that develops either from the endocardial cushions or as an extension of the trabecular septum), and the conal septum. Fusion of the superior and inferior atrioventricular cushions and mesenchymal cap of the primary atrial septum forms the membranous septum. Septation is completed in the normal heart when the aorta transfers over to the left ventricle and wedges itself next to the forming mitral valve (Fig. 30.1B) (16).
outlets (conus) (15). The muscular portion of the ventricular septum is formed from the trabecular septum (between the embryonic left and right ventricles), the inlet component (that develops either from the endocardial cushions or as an extension of the trabecular septum), and the conal septum. Fusion of the superior and inferior atrioventricular cushions and mesenchymal cap of the primary atrial septum forms the membranous septum. Septation is completed in the normal heart when the aorta transfers over to the left ventricle and wedges itself next to the forming mitral valve (Fig. 30.1B) (16).
Morphology
Pathologic Anatomy
Understanding the anatomy of the ventricles and ventricular septum is crucial to accurate description and classification of VSDs. In the setting of concordant ventriculoarterial connections, each ventricle can be divided into three segments using the tripartite approach (17), and these segments are the inlet, apical (trabecular), and outlet components. The right ventricular outlet is composed of the subpulmonary conus (also known as infundibulum), which is the muscular chamber preventing fibrous continuity between the tricuspid and pulmonary valves (Fig. 30.2A). In contrast, the subaortic conus in the left ventricle regresses during normal gestation, resulting in fibrous continuity between the mitral and aortic valves (Fig. 30.2B). Most early reports looking at VSDs divide the right ventricular septal surface into distinct regions: the inlet septum, the membranous septum at the base of the heart, the muscular trabecular septum, and the outlet or conal septum separating the subarterial regions (see Fig. 30.2A) (18). The septal band, also known as the septomarginal trabeculation, is a Y-shaped right ventricular septal structure that represents one component of the separation between the apical trabecular component of the right ventricle and the right ventricular conus. The superior aspect of the septal band divides into an anterosuperior limb extending and merging into the subpulmonary conus and a posteroinferior limb extending toward the inlet septum. The conal septum is usually situated within the space bordered by the two limbs of the septal band.
The anatomic classification of VSDs has been fraught with controversy, and many approaches have been reported in the literature (18,19,20,21,22,23,24). The majority of these classification systems belong to one of two categories: the geographic approach focuses primarily on the location of the defect within the ventricular septum, and the border approach focuses primarily on the anatomic structures immediately adjacent to the defect.
Early reports using the geographic approach based on pathologic specimens have divided VSDs into defects involving the ventricular outflow tracts and those that are distant from the outflow tracts, the former found to be more common than the latter (19). Outflow tract defects are further subdivided based on whether they are located posteriorly or anteriorly to the crista supraventricularis (supraventricular crest), likely the origin of the terms “infracristal” and “supracristal” used early on to classify VSDs. Subsequent reports have utilized specified areas of the right ventricular aspect of the ventricular septum to classify the defects, and most of these have divided the right ventricular septal surface into the distinct regions shown below: the inlet septum, the membranous septum, the muscular trabecular septum, and the conal septum (see Fig. 30.2A) (18). Within this framework, defects are classified as inlet, membranous, muscular, or outlet VSDs; the inlet defect can be associated with malalignment between the atrial septum and ventricular septum, and the outlet defect can be associated with malalignment between the conal septum and remainder of the muscular ventricular septum. Further refinement of this classification system equates inlet VSDs to VSDs of the atrioventricular canal-type and outlet VSDs to conal septal defects (21). Within this system, conoventricular defects encompass membranous defects as well as those involving malalignment of the conal septum. More recently, the Society of Thoracic Surgeons utilized the following classification system for its database project: type 1 (subarterial), type 2 (perimembranous), type 3 (inlet), and type 4 (muscular) (23).
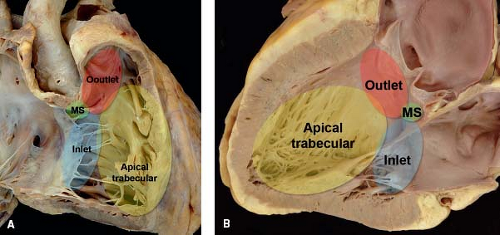 Figure 30.2 Pathologic specimen of a normal heart depicting the inlet (blue area), apical trabecular (yellow area), and outlet (red area) segments that represent the three components of the tripartite ventricle from the perspective of (A) the right-sided cardiac structures, and (B) the left-sided cardiac structures. In both images, the membranous septum (green area) represents the area of fibrous continuity between the tricuspid and aortic valves where offsetting between the tricuspid and mitral valves occur; this is the location where the atrioventricular node penetrates atrioventricular septum and connects to the bundle of His (MS, membranous septum). (From the Web Portal of the Archiving Working Group of The International Society for Nomenclature of Paediatric and Congenital Heart Disease (ISNPCHD) (http://ipccc-awg.net) and courtesy of Diane E. Spicer BS, PA(ASCP), The Congenital Heart Institute of Florida, St Petersburg, USA.) |
An alternative approach has focused on the borders of the defect, namely the atrioventricular valves, membranous septum, muscular ventricular septum, and semilunar valves, with specific attention to the areas of fibrous continuity between the atrioventricular and semilunar valves (20,22). Perimembranous VSDs are located in the area of fibrous continuity between the tricuspid and aortic valves. The septal leaflet of the tricuspid valve represents the posterior border of the defect, and the right and/or noncoronary leaflets of the aortic valve represent the adjacent anterosuperior border. These defects can extend into the inlet septum, into the muscular trabecular septum, and into the outlet. In one report, the term conoventricular defect is used instead of membranous or perimembranous VSD, defining it as a defect located between the limbs of the septal band and frequently associated with abnormalities of the conal septum (22). Not surprisingly, muscular VSDs are completely bordered by muscular ventricular tissue and may be located in the inlet, muscular trabecular, or outlet septum. Subarterial VSDs are associated with absent conal septum so that they are in fibrous continuity with both the aortic and pulmonary valves.
In the context of this debate, extensive work by the International Society for the Nomenclature of Paediatric and Congenital Heart Disease has resulted in the definition of a VSD as a congenital cardiac malformation in which there is a hole or pathway between the ventricular chambers or ventricular remnants. The proposed classification scheme is essentially a geographic approach dividing VSDs into four types—central VSD, inlet VSD, muscular trabecular VSD, and outlet VSD—highlighting the importance of the borders or margins of the defect to facilitate understanding of the definition of each category (Table 30.1).
Central VSDs
Central VSDs are located at the base of the heart in the area of the membranous ventricular septum. One margin of the defect almost always involves the area of fibrous continuity between an atrioventricular valve and a semilunar valve, which in hearts with concordant ventriculoarterial connections is the area where the tricuspid and aortic valves are in fibrous continuity. Hence these defects are located behind the septal leaflet of the tricuspid valve and below the right and/or noncoronary leaflets of the aortic valve (Fig. 30.3). In some reports, these VSDs have been further limited in their location to the area below the posteroinferior limb of the septal band, distinguishing them from outlet defects, which are located between the two limbs of the septal band (25). This distinction, however, still remains somewhat controversial. Central VSDs are often partially covered by accessory (possibly) tricuspid valve tissue, contributing to restrictive flow across the defect or to spontaneous closure of the defect. Because the defect is adjacent to the aortic valve, the right or noncoronary leaflet occasionally prolapses into the defect as a result of the Venturi effect with consequent aortic regurgitation. In some circumstances, there is a small rim of muscle separating the defect from the aortic valve. In these cases, prolapse of the aortic valve leaflet is less likely. Other terms that have been used for this defect include membranous VSD, perimembranous VSD, paramembranous VSD, type 2 VSD, and conoventricular defect without conal septal malalignment as well as less useful terms such as infracristal and subaortic VSDs. Based on phenotypic differences, defects labeled as perimembranous VSDs with posterior extension into the inlet septum have been classified as inlet VSDs, and defects labeled as perimembranous VSDs with anterior extension into the outlet septum as well as conoventricular defects with or without malalignment of the conal septum have been classified as outlet VSDs (25).
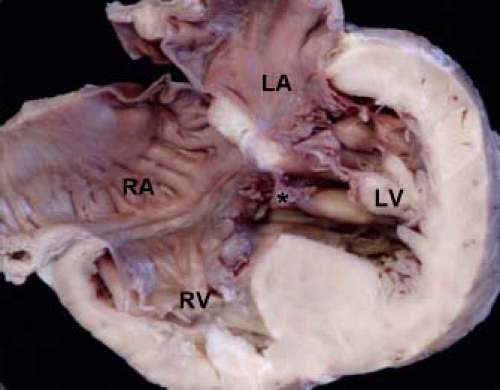
The segment of the membranous septum where the tricuspid valve is normally offset from the mitral valve (also known as the atrioventricular component of the membranous septum because it is continuous with the right atrium in this area) is an important structure, because this is the location of the penetrating bundle of the atrioventricular
node (Fig. 30.4) (26). A deficiency of this segment can result in a shunt from the left ventricle to the right atrium, a lesion known as a Gerbode defect (27). An extremely rare type of VSD, this lesion should be distinguished from central VSDs wherein the VSD jet courses through accessory tricuspid valve tissue directly into the right atrium.
node (Fig. 30.4) (26). A deficiency of this segment can result in a shunt from the left ventricle to the right atrium, a lesion known as a Gerbode defect (27). An extremely rare type of VSD, this lesion should be distinguished from central VSDs wherein the VSD jet courses through accessory tricuspid valve tissue directly into the right atrium.
TABLE 30.1 VSD Classification | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
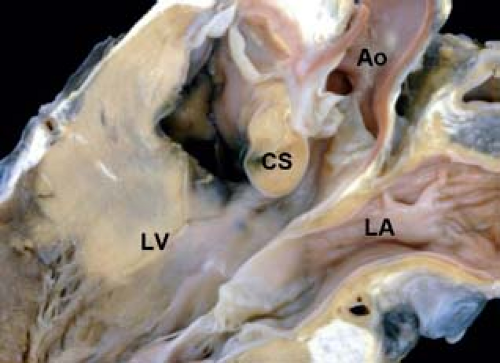
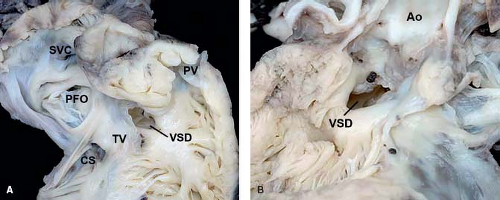 Figure 30.3 Pathologic specimen of a central ventricular septal defect as viewed from (A) the right ventricle and (B) the left ventricle; the defect is bordered by the area of fibrous continuity between the tricuspid and aortic valves, and it is located behind the septal leaflet of the tricuspid valve and below the aortic valve, extending into the apical trabecular muscular septum (Ao, aorta; CS, coronary sinus; PFO, patent foramen ovale; PV, pulmonary valve; SVC, superior vena cava; TV, tricuspid valve; VSD, ventricular septal defect). (From the Web Portal of the Archiving Working Group of The International Society for Nomenclature of Paediatric and Congenital Heart Disease (ISNPCHD) (http://ipccc-awg.net) and courtesy of Diane E. Spicer BS, PA (ASCP), The Congenital Heart Institute of Florida, St Petersburg, USA.) |
Inlet VSDs
Inlet VSDs are those defects opening into the inlet component of the right ventricle and extending along the length of the tricuspid valve (Fig. 30.5) (28,29). They can be divided into inlet muscular defects with exclusively muscular borders (such that the superior rim of the defect is muscular rather than atrioventricular valvar tissue) (Fig. 30.6) and inlet perimembranous defects, with the latter category representing those defects whose posteroinferior border involves the area of fibrous continuity between an atrioventricular valve and a semilunar valve (see Fig. 30.5B). Inlet perimembranous VSDs are further subdivided into those with alignment of the atrial septum and ventricular septum and those with malalignment associated with straddling and/or overriding of the tricuspid valve (Fig. 30.7). Inlet VSDs with distinct tricuspid and mitral valves must be distinguished from variants of atrioventricular septal defects with a common atrioventricular valve, no significant shunting across the atrial component of the defect, and shunting exclusively through the ventricular component of the defect (28). Other terms that have been used for the inlet defect include atrioventricular canal-type VSD, perimembranous VSD with posterior (inlet) extension, and type 3 VSD.
Muscular (Trabecular) VSDs
Muscular (trabecular) VSDs have exclusively muscular borders, are the least controversial with regard to nomenclature, and are synonymous with type 4 VSDs. They are located within the more apically located segments of the muscular ventricular septum. They are further classified based on their location within the muscular septum, and the most common descriptors of muscular VSDs in current use involve
the midseptal (Fig. 30.8A), apical, anterior, and inferior (posterior) areas of the septum (Fig. 30.8B). Small muscular VSDs are frequently seen in the newborn period, and many close spontaneously over time without intervention. Because of the coarse trabeculations along the right ventricular surface of the septum, the channel can often be complex with a single entrance on the left ventricular side and multiple exits on the right ventricular side. In addition, multiple muscular VSDs can occur in aggregate, resulting in a “Swiss-cheese” type of ventricular septum, often with significant interventricular shunting.
the midseptal (Fig. 30.8A), apical, anterior, and inferior (posterior) areas of the septum (Fig. 30.8B). Small muscular VSDs are frequently seen in the newborn period, and many close spontaneously over time without intervention. Because of the coarse trabeculations along the right ventricular surface of the septum, the channel can often be complex with a single entrance on the left ventricular side and multiple exits on the right ventricular side. In addition, multiple muscular VSDs can occur in aggregate, resulting in a “Swiss-cheese” type of ventricular septum, often with significant interventricular shunting.
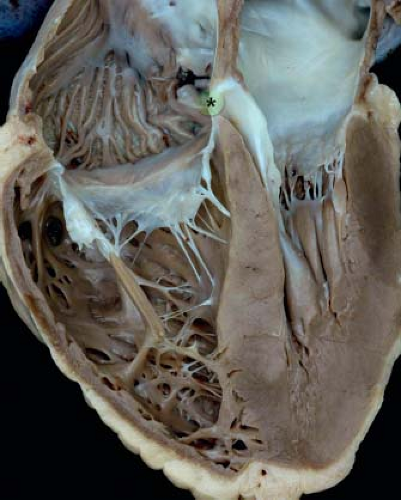 Figure 30.4 Pathologic specimen sectioned to reveal the four cardiac chambers as well as the area of offsetting between the tricuspid and mitral valves (star and green area) representing the atrioventricular component of the membranous septum. (From the Web Portal of the Archiving Working Group of The International Society for Nomenclature of Paediatric and Congenital Heart Disease (ISNPCHD) (http://ipccc-awg.net) and courtesy of Diane E. Spicer BS, PA(ASCP), The Congenital Heart Institute of Florida, St Petersburg, USA.) |
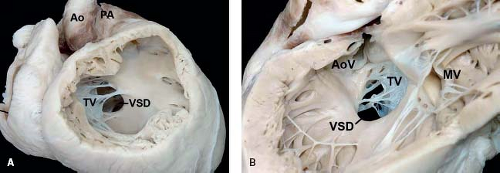 Figure 30.5 Pathologic specimen of an inlet perimembranous ventricular septal defect as viewed from (A) the right ventricle and (B) the left ventricle; the defect is continuous with and extends along the length of the septal leaflet of the tricuspid valve, and its posteroinferior border involves the area of fibrous continuity between the tricuspid and aortic valves (Ao, aorta; AoV, aortic valve; MV, mitral valve; PA, pulmonary artery; TV, tricuspid valve; VSD, ventricular septal defect). (From the Web Portal of the Archiving Working Group of The International Society for Nomenclature of Paediatric and Congenital Heart Disease (ISNPCHD) (http://ipccc-awg.net) and courtesy of Diane E. Spicer BS, PA(ASCP), The Congenital Heart Institute of Florida, St Petersburg, USA.) |
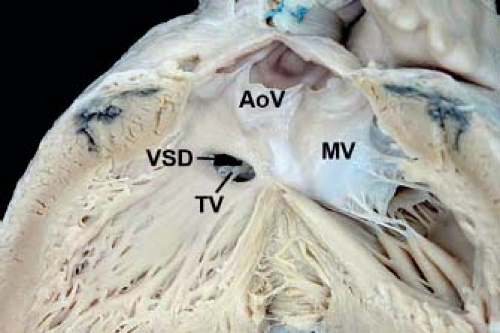 Figure 30.6 Pathologic specimen of an inlet muscular ventricular septal defect as viewed from the left ventricle with a muscular separation between the defect and the aortic valve leaflets (AoV, aortic valve; MV, mitral valve; TV, tricuspid valve; VSD, ventricular septal defect). (From the Web Portal of the Archiving Working Group of The International Society for Nomenclature of Paediatric and Congenital Heart Disease (ISNPCHD) (http://ipccc-awg.net) and courtesy of Diane E. Spicer BS, PA(ASCP), The Congenital Heart Institute of Florida, St Petersburg, USA.) |
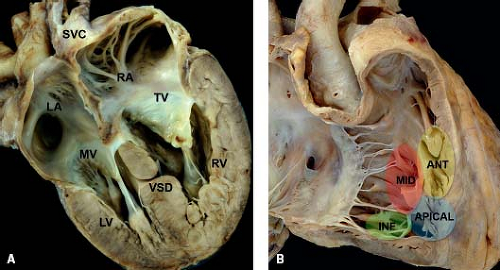 Figure 30.8 (A) Pathologic specimen depicting a midmuscular ventricular septal defect in a four-chamber view from a posterior perspective (LA, left atrium; LV, left ventricle; MV, mitral valve; RA, right atrium; RV, right ventricle; SVC, superior vena cava; TV, tricuspid valve; VSD, ventricular septal defect); (B) pathologic specimen of a normal heart depicting the standard locations used for muscular ventricular septal defects, specifically the midseptal (red area), apical (blue area), anterior (yellow area), and inferior (posterior) (green area) segments of the muscular ventricular septum (ANT, anterior; INF, inferior; MID, midseptal). (From the Web Portal of the Archiving Working Group of The International Society for Nomenclature of Paediatric and Congenital Heart Disease (ISNPCHD) (http://ipccc-awg.net) and courtesy of Diane E. Spicer BS, PA(ASCP), The Congenital Heart Institute of Florida, St Petersburg, USA.) |
Outlet VSDs
Outlet VSDs open into the outlet component of the right ventricle and almost never close spontaneously. In most classification systems, these VSDs involve only those defects with absence or hypoplasia of the conal septum, also known as infundibular VSD, subarterial infundibular VSD, doubly committed subarterial VSD, conal septal VSD, intraconal VSD, and type 1 VSD. Less useful synonyms have included subpulmonic VSD and supracristal VSD. When the conal septum is absent or is present only as a purely fibrous structure, the cranial border of the defect is the area of fibrous continuity between the pulmonary and aortic valves, and these defects are also known as doubly committed juxta-arterial VSDs (Fig. 30.9). The absence of supporting musculature below the right coronary leaflet of the aortic valve can result in prolapsing of the leaflet through the defect into the right ventricle, and the consequent distortion of the aortic valve can result in aortic regurgitation. These defects can extend posteroinferiorly into the area of fibrous continuity between the tricuspid and aortic valves
(in the setting of ventriculoarterial concordance) resulting in a confluent outlet and central VSD. When the conal septum is hypoplastic, the defect is in the location of the conal septum but has muscular borders along its entire circumference without fibrous continuity between the defect and the pulmonary valve.
(in the setting of ventriculoarterial concordance) resulting in a confluent outlet and central VSD. When the conal septum is hypoplastic, the defect is in the location of the conal septum but has muscular borders along its entire circumference without fibrous continuity between the defect and the pulmonary valve.
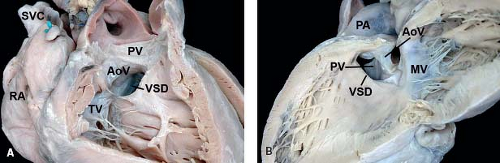 Figure 30.9 Pathologic specimen of an outlet ventricular septal defect as viewed from (A) the right ventricle and (B) the left ventricle; the conal septum is absent, and the cranial border of the defect is the area of fibrous continuity between the pulmonary and aortic valves (AoV, aortic valve; MV, mitral valve; PA, pulmonary artery; PV, pulmonary valve, RA, right atrium; SVC, superior vena cava; TV, tricuspid valve; VSD, ventricular septal defect). (From the Web Portal of the Archiving Working Group of The International Society for Nomenclature of Paediatric and Congenital Heart Disease (ISNPCHD) (http://ipccc-awg.net) and courtesy of Diane E. Spicer BS, PA(ASCP), The Congenital Heart Institute of Florida, St Petersburg, USA.) |
Malalignment VSDs
Defects where the conal septum has rotated out of the plane of the limbs of the septal band, thereby resulting in malalignment between the conal septum and the remainder of the muscular ventricular septum, can be considered outlet VSDs, though some have utilized a separate and distinct category for these lesions and have labeled them as conoventricular defects or malalignment VSDs (Fig. 30.10). Outlet VSDs with conal septal malalignment usually occur in the setting of other congenital cardiac malformations. For example, anterior malalignment of the conal septum in a heart with ventriculoarterial concordance is usually seen in the setting of tetralogy of Fallot, where the anteriorly malaligned or malrotated conal septum is associated with underdevelopment of the right ventricular conus as well as obstruction along the subpulmonary region. In milder forms of anterior malalignment without significant subpulmonary obstruction, this type of defect has been called an Eisenmenger-type VSD. The importance of defining it as such is that an Eisenmenger-type VSD does not close spontaneously. Posterior malalignment of the conal septum is usually seen in association with subaortic stenosis and aortic arch obstruction or interruption (see Fig. 30.10). Posterior malalignment of the conal septum can be subtle but also means that the defect will not close on its own. Defects can also be seen in combination. For example, malalignment defects can be associated with absence of the conal septum.
Conduction
Correct identification of the type of VSD is especially important if the patient will require surgical intervention, because the type of VSD usually predicts the location of ventricular conduction tissue (26). As previously discussed, the penetrating bundle of the atrioventricular node is located in the atrioventricular component of the membranous septum above the level of the tricuspid valve (see Fig. 30.4). Thus, surgical intervention must assure that sutures are not placed within this region. In the central or perimembranous VSD, the atrioventricular conduction axis is always found along the posteroinferior border of the defect at or near the crest of the muscular ventricular septum. When a central VSD extends posteriorly into the inlet septum, the bundle is located more closely to the crest of the septum than when the central VSD extends anteriorly into the outlet septum. In the inlet muscular VSD without anterior extension into the area of the membranous septum, the atrioventricular conduction axis is located along the anterosuperior border of the defect, a distinctly different location to that seen in central VSDs (28). Muscular trabecular VSDs and outlet VSDs without extension into the area of the membranous septum are almost always distant from the atrioventricular conduction axis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree