Congenital Anomalies of the Coronary Vessels and the Aortic Root
D. Scott Lim
G. Paul Matherne
Coronary and aortic root anomalies represent a relatively small but interesting group of malformations that may occur alone or in association with structural heart disease (1,2,3). Recognizing and identifying these anomalies has become an important part of the evaluation of complex congenital heart disease. In the absence of structural heart disease, coronary anomalies are also important in certain clinical situations such as dilated cardiomyopathy (4), hypertrophic cardiomyopathy (5), and sudden cardiac events in older children (6). This chapter will review coronary artery development and anatomy, coronary anomalies in the absence of structural heart disease, coronary anomalies in the presence of structural heart disease, and aortic root anomalies.
Coronary Vascular Anomalies
Embryology
The cells of the developing myocardium initially receive nourishment directly from circulating blood in the ventricular cavity. As the myocardium thickens and develops, the presence of multiple trabeculations allows close proximity of the myocardial cells to the ventricular cavity. These trabeculations then develop into a sinusoidal system that continues to minimize diffusion distance between the myocytes and the circulation. While previously these sinusoids were thought to be the forerunners of the coronary vascular system, but new data have provided evidence for an epicardial origin of the coronary vascular system (7).
The new model of coronary vascular development (7) begins with formation of a proepicardial profusion by cells from the primordial liver. These cells establish the proepicardium and epicardial cells and then migrate over the surface of the heart. The epicardial cells invade the forming subepicardial matrix and form the coronary vascular plexus. The epicardial cells then undergo epithelial mesenchymal transformation by an as yet undefined mechanism that probably involves multiple growth factors. Nascent capillaries then are associated with subepicardial mesenchymal cells to form mature vessels, which fuse and grow inward to penetrate the aorta rather than coronary buds from the aortic sinuses fusing with the coronary vessels (8).
The new experimental data on the development of the coronary system implicate multiple growth factors as well as adhesion molecules and chemotactic factors in this complicated coordinated migration and transformation of cells to form coronary vessels. The presence of congenital anomalies of coronary arteries suggests abnormalities in these signaling pathways or alterations in local factors that direct coronary vessel development.
Anatomy
Coronary Arteries
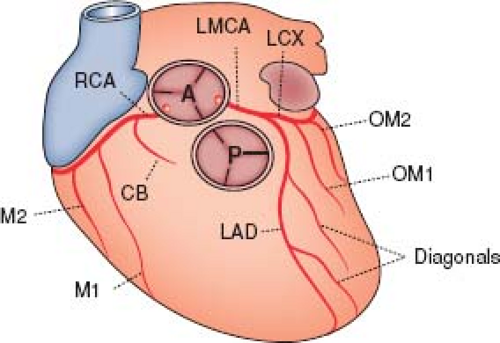
Normal coronary artery anatomy will be briefly reviewed, but for a complete discussion on this subject the reader is referred to Frank Netter’s diagrams in the CIBA Collection of Medical Illustrations (9). The entire blood flow to the myocardium is derived from two main coronary arteries arising from the right and left aortic sinuses of Valsalva (Fig. 32.1). The left main coronary artery is of variable length in adults (average 13 mm long, range 2 to 40 mm) and gives rise to the circumflex branch, which courses posteriorly in the atrioventricular groove; the left main coronary then continues as a left anterior descending branch. The right coronary artery gives rise to a small conal branch and then courses posteriorly in the opposite direction along the atrioventricular groove. There is no separate septal branch, the septum being supplied by perforating branches that enter the septum from the anterior and posterior descending coronary arteries. In 69% of the population, the right coronary artery is dominant (10), giving rise to the posterior descending coronary artery, which extends to the apex and supplies the posterior part of the ventricular septum, the inferior wall of the left ventricle, and the atrioventricular node (11). In 11% the left coronary artery is
dominant, thereby giving rise to the posterior descending coronary artery, and in 20% there is codominance (10). The left coronary arterial system supplies the free wall of the left ventricle. Interestingly, a significant number of patients with bicuspid aortic valves or aortic stenosis (20% to 57%) have left dominant systems and a short left main coronary artery (10,12,13).
dominant, thereby giving rise to the posterior descending coronary artery, and in 20% there is codominance (10). The left coronary arterial system supplies the free wall of the left ventricle. Interestingly, a significant number of patients with bicuspid aortic valves or aortic stenosis (20% to 57%) have left dominant systems and a short left main coronary artery (10,12,13).
Within the myocardium, small arteries branch repeatedly until they reach the endocardium. Normally, there are connections between coronary arterial branches that are 25 to 200 μm in diameter and are known as collaterals. They may be superficial or subendocardial, and they are capable of enlarging if pressure gradients develop between branches. These collateral arteries become significant in cases of primary arterial occlusion, in which cases they may allow reperfusion of affected myocardium.
Cardiac Veins
The coronary sinus arises from the proximal portion of the left sinus horn and the common cardinal vein. The great cardiac vein begins at the apex and runs up the anterior interventricular groove to enter the coronary sinus before running around the left edge of the heart in the posterior atrioventricular groove until it enters the right atrium near the atrioventricular node. The middle cardiac vein runs up in the posterior atrioventricular groove to enter the coronary sinus. The posterior ventricular vein drains the free wall of the left ventricle up to the coronary sinus. The small cardiac vein runs with the right coronary artery in the right posterior part of the atrioventricular groove; it drains into the coronary sinus or directly into the right atrium, as do the small veins draining the right ventricular free wall (14,15).
Anomalies of Coronary Arteries in the Absence of Structural Heart Disease
Normal Variations
The right and left coronary arteries arise from the right and left aortic sinuses of Valsalva (see Fig. 32.1). Usually they come from the middle of the sinuses, but they may arise from the sinotubular junction or even above it. The position of the ostium does not appear to affect the flow through it. The ostia may be round, oval, or elliptical. The arteries are usually perpendicular to the aortic wall; that is, they are radially arranged relative to the center of the aorta.
Separate origin of the conus branch of the right coronary artery occurs commonly (11). The corresponding anomaly on the left side—separate origins of the left anterior descending and left circumflex coronary arteries—occurs in about 1% of people and is more frequent with bicuspid aortic valves (11). Neither of these anomalies appears to have any clinical consequences.
Abnormal Origin of Right or Left Coronary Artery from Inappropriate Sinus
Anomalous Origin of Left Coronary Arterial Branches from Right Sinus of Valsalva
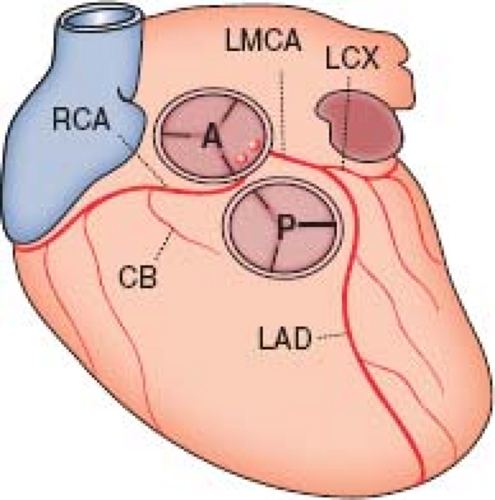
The most common anomaly, accounting for about one-third of all major coronary arterial anomalies, is origin of the left circumflex coronary artery from the right main coronary artery (Fig. 32.2A) (2,3,16,17). The left circumflex coronary artery passes behind the aorta to reach its normal territory of supply. This anomaly has no general clinical significance in the pediatric population, but the artery may be compressed if both mitral and aortic prosthetic valves or annuloplasty rings are implanted. These anomalous arteries may have an unusually high incidence of coronary atheroma (2).
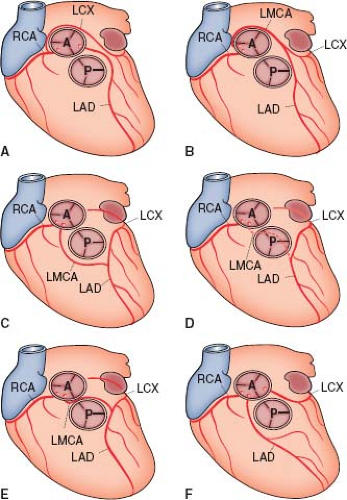
Much less common, accounting for 1% to 3% of major coronary arterial anomalies (2,16), but of greater clinical significance, is origin of the left main coronary artery from the right sinus of Valsalva (1,16,18). There are four pathways that the left main coronary artery can take after leaving the sinus: posterior to the aorta (Fig. 32.2B), anterior to the right ventricular outflow tract (Fig. 32.2C), within the ventricular septum beneath the right ventricular infundibulum (Fig. 32.2D, the most common variant), and between the aorta and the right ventricular outflow tract (Fig. 32.2E). With rare exceptions, the first three courses have not been associated with sudden death or premature myocardial ischemia. The course that passes between the two great arteries, however, has often been associated with sudden death in people during or just after vigorous exercise. Several of these patients had had episodes of syncope or anginal chest pain during previous exercise. In most of these patients, the ostium of the left main coronary artery was slitlike, with an intramural course within the aortic root and adherent to it for about 1.5 cm (18).
In some patients, the left anterior descending coronary artery originates in the right sinus of Valsalva or from the right main coronary artery (Fig. 32.2F). This anomaly is rare in the absence of congenital heart disease (2,18) but is common in tetralogy of Fallot. The artery usually passes in front of the right ventricular outflow tract or through the interventricular septum but has rarely been
seen to pass between the aorta and right ventricular outflow tract. Should there be atheroma near the ostium of the common arterial trunk, then most of the heart will become ischemic, so that the lesion is the equivalent of a left main coronary stenosis.
seen to pass between the aorta and right ventricular outflow tract. Should there be atheroma near the ostium of the common arterial trunk, then most of the heart will become ischemic, so that the lesion is the equivalent of a left main coronary stenosis.
Anomalous Origin of Right Coronary Arterial Branches from the Left Sinus of Valsalva
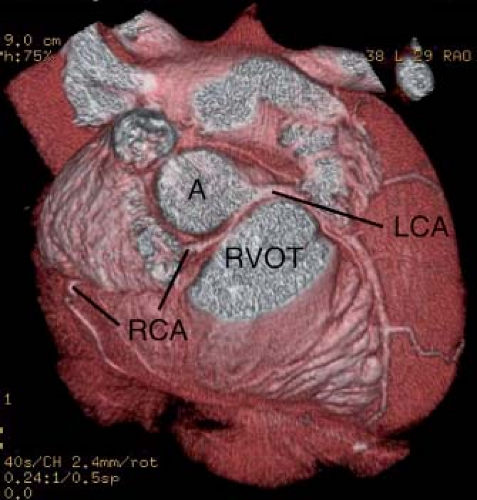
Origin of the right main coronary artery from the left sinus of Valsalva, first described by White and Edwards in 1948 (19), is relatively common, making up about 30% of all major coronary arterial anomalies (2,18), and has a significantly higher incidence in Asians and Hispanics (20). The right coronary artery then runs between the aorta and the right ventricular outflow tract to reach the right side of the atrioventricular groove, after which it is distributed normally (Figs. 32.3 and 32.4). This anomaly was once thought to be benign, but there are now many reports of myocardial ischemia, infarction, or sudden death (21,22,23). In many of the autopsies, the origin of the right main coronary artery was angulated and the ostium described as slitlike.
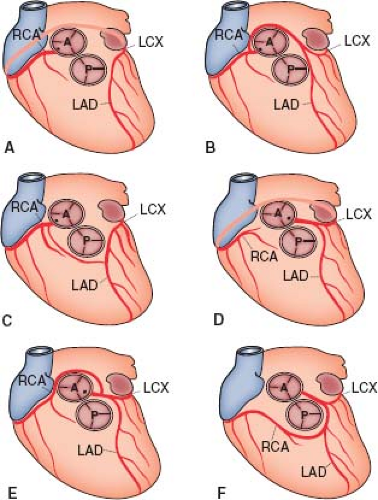
Single Coronary Artery
In 5% to 20% of major coronary arterial anomalies, a single coronary artery arises from the aorta and then branches (17,24). Sometimes an atretic cord connects part of the artery to a sinus of Valsalva that has no ostium. About 40% of these anomalies are associated with other cardiac malformations, including transposition of the great vessels, tetralogy of Fallot, truncus arteriosus, coronary-cameral fistulas, and bicuspid aortic valves. The single artery can arise from either the right (Fig. 32.5A–C) or the left sinus of Valsalva (Fig. 32.5D–F) with many variations (24). The single coronary artery on either side can follow its usual course and then
continue on to supply the other side of the heart, or separate branches can arise from a main coronary artery and course posteriorly or anteriorly to supply the other side of the heart. Branches also can pass between the great vessels.
continue on to supply the other side of the heart, or separate branches can arise from a main coronary artery and course posteriorly or anteriorly to supply the other side of the heart. Branches also can pass between the great vessels.
For the single coronary arteries arising from the right side, the right coronary can follow the course of the normal right coronary artery and continue as the left circumflex artery, which then gives off the left anterior descending coronary artery (see Fig. 32.5A). Alternatively, after the right coronary artery arises, a separate branch to the left side can arise that passes posterior to the aorta and gives rise to a circumflex vessel and a left anterior descending coronary artery (see Fig. 32.5B), or the separate branch can follow a course anterior to the right ventricular infundibulum, giving rise to the anterior descending coronary artery before continuing on as the circumflex (see Fig. 32.5C).
A single left coronary artery can display branching patterns similar to those on the right. A single left coronary artery can branch into the left anterior descending and left circumflex coronary arteries with the circumflex continuing across the crux to form the right coronary artery (see Fig. 32.5D). A separate right coronary vessel also can arise from the single left coronary artery and pass posterior to the aorta (see Fig. 32.5E) to reach the opposite side of the heart, or the vessel can pass anterior to the right ventricular infundibulum (see Fig. 32.5F). Most single coronary arteries produce no symptoms in the absence of severe atheroma (which is clearly more serious when there is only one main artery supplying the entire myocardium), but a small number of premature deaths have been reported with this anomaly (24). It is usually those variants in which a major branch passes between the aorta and the right ventricular infundibulum that are at greatest risk for sudden death (24), but other patterns can cause myocardial ischemia.
Left or Right Coronary Arterial Branches Arising from the Posterior Sinus of Valsalva
These are very rare (18) and have not been associated with premature or sudden death.
Pathology and Clinical Features of Abnormal Origin of Right or Left Coronary Artery from Inappropriate Sinus
Pathology
In about 20% of autopsies there are subendocardial scars, and occasionally a major myocardial territory infarction is reported. However, the suddenness of death in most of these patients prevents large scar from occurring. Occasionally, severe atherosclerosis has been seen in a segment of the abnormal vessels, even in children (25). In some of the anomalies, the initial few millimeters of artery may run within the aortic wall. Finally, the anomalous artery may arise tangentially from the aorta, and its ostium may be slitlike and partly covered by a valvelike flap.
Mechanisms of Death
Death is almost certainly due to myocardial ischemia, but the exact mechanism is unknown. The left ventricular myocardium has a huge demand for oxygen during strenuous exercise. Systolic pressure increases during strenuous exercise, and there is activation of the sympathetic nervous system. The root of the aorta therefore distends in systole. If part of the anomalous artery runs within the wall, it may be compressed, and if the artery runs adjacent to the wall, it may be stretched, compressed, or both. Presumably, the severe myocardial ischemia that occurs from any of these mechanisms produces either ventricular fibrillation or electromechanical dissociation. In those with previous syncope, the severe ischemia might have produced transient ventricular tachycardia or fibrillation, or else suddenly impaired ventricular function might have decreased cardiac output catastrophically. Why some patients with apparently identical anomalies survive without ischemia until their 70s and 80s is unknown.
Clinical Features
Most of these anomalous arteries do not cause myocardial ischemia, particularly if the anomalous branch does not pass between the aorta and the right ventricular infundibulum. Even those that do run between these structures do not always lead to sudden or premature death. However, this is the group that accounts for symptoms and sudden death in children, adolescents, and young adults. Although the first sign of the anomaly is sometimes sudden death or a fatal myocardial infarction, in many of these patients there may be a history of syncope or prolonged chest pain before the fatal event. These symptoms almost invariably come on during or just after strenuous exercise, and many of the victims have been athletes.
Diagnosis
Any episode of syncope or of severe chest pain during or after exercise calls for intensive investigation. The standard clinical examination usually shows no abnormalities. A resting electrocardiogram should be performed to look for ventricular hypertrophy, evidence of prior infarction, and persistent arrhythmia; an echocardiogram should be performed to exclude persisting ventricular dysfunction, hypertrophic cardiomyopathy, and proximal coronary anatomy. Careful attention should be directed to the origins of the coronary arteries because most of the anomalies affect the origins of the major arteries or their major branches and therefore may be detectable by echocardiography. Following the course of the coronary vessels is also important because the lesions with the highest risk of sudden death are associated with a major branch passing between the great vessels. Because most patients are older children or adults, the resolution of the transthoracic echocardiogram may be inadequate to show the anomalies, and transesophageal echocardiography (26), magnetic resonance imaging (27), or computed tomographic scans (28) (see Fig. 32.4) may be more sensitive.
Evaluating blood pressure and the electrocardiogram or injecting thallium at near-maximal exercise can be useful. However, a normal near-maximal stress test result has been reported in patients who subsequently died suddenly and had an anomalous left main coronary artery (29). Because of this, exertional syncope or severe exertional chest pain in a child or young adult warrants further investigation if the echocardiogram is inconclusive.
Anomalous Left Coronary Artery from the Pulmonary Artery
In this anomaly the left coronary artery arises from the pulmonary artery, usually from the left posterior facing sinus (Fig. 32.6) (Video 32.1). This anomaly was first described by pathologists in 1866 (30), and by 1962 Fontana and Edwards (31) had collected descriptions of 58 necropsies with this anomaly; most of these patients died at less than 13 months of age. The first report relating clinical and autopsy findings in a 3-month-old boy was by Bland et al. (32). The anomaly has thus been called the Bland–White–Garland syndrome.
Pathophysiology
In fetal life, this anomaly probably has no harmful effect: pressures and oxygen saturations are similar in the aorta and pulmonary
artery. Myocardial perfusion is presumably normal, and there is no stimulus to collateral formation (see Fig. 32.6A). After birth, however, the pulmonary artery contains desaturated blood at pressures that rapidly fall below systemic pressures. Therefore, the left ventricle, with its huge demand for oxygen, is perfused with desaturated blood at low pressures. Collateral flow is initially low. The left ventricular myocardial vessels dilate to reduce their resistance and increase flow, but soon coronary vascular reserve becomes exhausted and myocardial ischemia ensues. At first, ischemia is transient and occurs only with exertion such as feeding or crying, but further increases in myocardial oxygen demand lead to infarction of the anterolateral left ventricular free wall (see Fig. 32.6B), with resultant compromise of left ventricular function. This causes congestive heart failure, which is often made worse by mitral regurgitation secondary to a dilated mitral valve annulus or infarction and dysfunction of the anterolateral papillary muscle. Collateral vessels between the normal right and abnormal left coronary artery enlarge, and with the increased flow so does the right coronary artery itself (see Fig. 32.6C; Video 32.1). However, because the left coronary artery is connected to the low-pressure pulmonary artery, the collateral flow tends to pass into the pulmonary artery rather than into the higher resistance myocardial blood vessels; there is a pulmonary–coronary steal with a left-to-right shunt. The shunt is usually relatively small in terms of cardiac output but relatively large in terms of coronary flow. In about 15% of these patients, myocardial blood flow can sustain myocardial function at rest or even during exercise. These are the patients who reach adult life (33).
artery. Myocardial perfusion is presumably normal, and there is no stimulus to collateral formation (see Fig. 32.6A). After birth, however, the pulmonary artery contains desaturated blood at pressures that rapidly fall below systemic pressures. Therefore, the left ventricle, with its huge demand for oxygen, is perfused with desaturated blood at low pressures. Collateral flow is initially low. The left ventricular myocardial vessels dilate to reduce their resistance and increase flow, but soon coronary vascular reserve becomes exhausted and myocardial ischemia ensues. At first, ischemia is transient and occurs only with exertion such as feeding or crying, but further increases in myocardial oxygen demand lead to infarction of the anterolateral left ventricular free wall (see Fig. 32.6B), with resultant compromise of left ventricular function. This causes congestive heart failure, which is often made worse by mitral regurgitation secondary to a dilated mitral valve annulus or infarction and dysfunction of the anterolateral papillary muscle. Collateral vessels between the normal right and abnormal left coronary artery enlarge, and with the increased flow so does the right coronary artery itself (see Fig. 32.6C; Video 32.1). However, because the left coronary artery is connected to the low-pressure pulmonary artery, the collateral flow tends to pass into the pulmonary artery rather than into the higher resistance myocardial blood vessels; there is a pulmonary–coronary steal with a left-to-right shunt. The shunt is usually relatively small in terms of cardiac output but relatively large in terms of coronary flow. In about 15% of these patients, myocardial blood flow can sustain myocardial function at rest or even during exercise. These are the patients who reach adult life (33).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree