Chapter 17 Ventricular Septal Defect
In 1879, Henri Roger wrote, “A developmental defect of the heart occurs from which cyanosis does not ensue in spite of the fact that a communication exists between the cavities of the two ventricles and in spite of the fact that admixture of venous blood and arterial blood occurs. This congenital defect, which is even compatible with a long life, is a simple one. It comprises a defect in the interventricular septum.”1
Roger went on to say that at necropsy, “The ventricular walls show no alteration, but in the upper portion of the interventricular septum beneath the mitral valve is an orifice which establishes a communication between the two ventricles.”1 And still further, “Among the congenital defects of the heart compatible with life and perhaps a long one, one of the most frequent which I have encountered … is the communication between the two ventricles because of failure of occlusion of the interventricular septum.”1
Ventricular septal defects remain the most common congenital malformation of the heart, occurring in 50% of children with congenital heart disease.2 Detection has increased dramatically with advances in imaging and with the facility of echocardiographic diagnosis in neonates, with spontaneous closure and detection of small defects in the muscular trabecular septum taken into account.3,4 A comprehensive review estimated the median incidence rate of ventricular septal defects to be 2,829 per million live births5; the defects are found to be the most common gross morphologic congenital malformation of the heart and circulation in adults, except for the bicuspid aortic valve.3,6–8
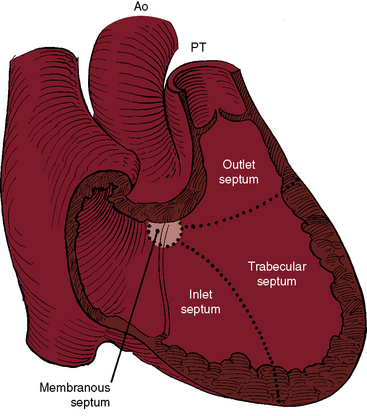
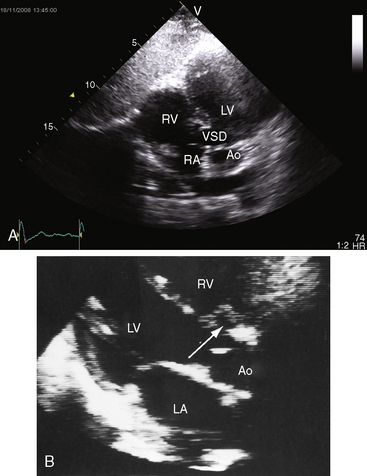
Although Roger believed that “this congenital defect … is a simple one,”1 there is still no uniform consensus on how best to characterize and classify the diverse types of defects to which the ventricular septum is subject. The ventricular septum is a complex nonplanar partition whose components are best defined according to anatomic landmarks on the right side of the septal surface (Figure 17-1).9–12 Defects are classified according to their relationship to the membranous, inlet, trabecular, and infundibular septum.2,12 The membranous septum is divided by the tricuspid annulus into ventriculoatrial and interventricular components that abut the three major segments of the muscular septum: namely, the inlet septum, which is lightly trabeculated; the trabecular septum, which is, as the name implies, heavily trabeculated; and the infundibular septum, which is nontrabeculated. The inlet septum is limited by the tensor attachments of the tricuspid valve and is so named according to its location. The trabecular septum lies between the inlet septum and the infundibular septum (see Figure 17-1). Atrioventricular conduction tissue penetrates the ventriculoatrial portion of the membranous septum. The His bundle and bundle branches run beneath the deficient interventricular component of the membranous septum close to the free edge of the ventricular septal defect.10,13
Approximately 80% of ventricular septal defects are perimembranous. The prefix peri underscores extension into adjacent portions of the inlet, trabecular, and infundubular septum. Large perimembranous defects encroach upon all three portions of the muscular septum (Figure 17-2A). Muscular ventricular septal defects are prevalent in neonates, with estimates as high as 53 of every 1000 live births. Approximately 90% of the defects close spontaneously within the first 10 months of life.
The most common types of muscular defects lie within the trabecular septum.10,14,15 These malformations are more apparent on the left septal surface and vary from small to large, from single to multiple (Figure 17-3), to a honeycombed or Swiss cheese–like structure with sieve-like fenestrations, to tortuous sinusoidal tracks threaded among septal trabeculae without through-and-through perforations.10,15 Sieve-like fenestrations or multiple small muscular defects have the net functional effect of a single large defect.
Isolated defects in the inlet septum represent approximately 8% of ventricular septal defects at surgery.16 An inlet ventricular septal defect that is bordered entirely by myocardium differs from an inlet defect that involves the basal portion of the septum near the crux where it is partially bordered by bridging atrioventricular valve tissue (see Chapter 15).10–12
The infundibular septum is represented by a small portion of muscle interposed between the outflow components of the left and right ventricles.17 A sleeve of subpulmonary infundibulum supports the leaflets of the pulmonary valve and separates the right ventricular outflow tract from the surface of the heart rather than from the left ventricular outflow track. Ventricular septal defects in the infundibular septum are also called supracristal, subpulmonary, subarterial, or doubly committed and account for approximately 5% to 7% of ventricular septal defects in North America and Western Europe, but for approximately 30% in Asian patients.14,16,17 Infundibular septal defects can be entirely muscular or can be partially rimmed by semilunar valve tissue (subarterial). Defects are considered doubly committed subarterial when little or no muscle is interposed between the outflow components of the left and right ventricles and when there is absence of the septal component of the subpulmonary infundibulum, so the aortic and pulmonary leaflets are in fibrous continuity.10,12,14,16,17 Doubly committed subarterial defects lie immediately beneath the valves of both arterial trunks, so the left and right coronary cusps of the aortic valve tend to prolapse into the outflow tract of the right ventricle (see section on Ventricular Septal Defect with Aortic Regurgitation). Much less commonly, a pulmonary cusp prolapses through the defect.18
Atrioventricular septal malalignment necessarily involves an inlet ventricular septal defect and is usually accompanied by straddling of the tensor apparatus of an atrioventricular valve that inserts (straddles) onto both sides of the ventricular septum.19 So-called Eisenmenger’s malalignment is represented by a perimembranous ventricular septal defect with anterior deviation of the infundibular septum.20 Not relevant to this chapter is malalignment between infundibular and trabecular septum that is associated with Fallot’s tetralogy (see Chapter 18) or much less commonly with coarctation of the aorta (see Chapter 8).
Henri Roger did not anticipate spontaneous closure. “The pathologic state of the heart existing before birth and consisting of an arrest of development is not susceptible to favorable changes, either by spontaneous evolution or by medical or surgical intervention.”1 However, in 1918, two reports speculated that ventricular septal defects might undergo spontaneous closure.21,22 One report was entitled “The Possibility of a Loud Congenital Heart Murmur Disappearing When a Child Grows Up.”21 The other report carried the title “Can the Clinical Manifestations of Congenital Heart Disease Disappear with the General Growth and Development of thePatient?”22 However, four decades elapsed before spontaneous closure was firmly documented.23–29 In 1960, Paul Wood wrote, “In any large series of geriatric necropsies … atrial septal defect is always well represented, but where’s the maladie de Roger? Assuming it does not provide immortality, it must either close spontaneously in middle life or have long since run its mortal course.”30
A defect may remain anatomically open but functionally closed (i.e., with absent or negligible shunt; Figures 17-2B and 17-4). The tendency for ventricular septal defects, especially perimembranous and trabecular muscular defects, to decrease in size finds an ultimate expression in complete closure24,31–33 that has been called the therapeutics of nature—the invisible sutures of spontaneous closure.34 The incidence rate of spontaneous closure varies considerably depending on the population under study, the method of diagnostic investigation, the type of defect, and whether or not the defect is solitary35 and has been estimated at 50% to 75% for restrictive perimembranous and trabecular muscular defects observed from birth.29,36,37 The incidence rate for trabecular muscular defects35 is reportedly equal to or somewhat higher than that for perimembranous defects. Moderately restrictive and nonrestrictive defects also close spontaneously, but the probability is comparatively low, with an incidence rate estimated at 5% to 10%.23–25,35,38,39 Most defects that are destined to close do so within the first year of life,35 with approximately 60% closing before 3 years of age and 90% closing before age 8 years.36,37 However, spontaneous closure also occurs in older children and young adults40 and has been documented at age 23 years,26 between 26 years and 33 years of age,41 and at age 46 years. Multiple muscular trabecular ventricular septal defects have a strong tendency to close, a conclusion in accord with the observation that multiple defects are three times more prevalent in neonates than after 1 year of age.42 In preterm infants, ventricular septal defects are about twice as frequent as in full-term infants, but the rate of spontaneous closure is the same,35 which calls into question the notion that defects reflect incomplete ventricular septation. Spontaneously closed defects in the muscular trabecular septum are funnel-shaped with a sealed orifice on the right ventricular aspect and residual patency on the left ventricular aspect, with endocardial proliferation in the lumen of the funnel and hypertrophy around the exit. Muscular defects represented by “Swiss cheese” fenestrations do not close spontaneously. Defects in the inlet septum seldom decrease in size43 but are occasionally occluded by bridging atrioventricular valve tissue (see Figure 15-87B). Infundibular defects can be closed by prolapse of the right aortic cusp.2 Perimembranous ventricular septal defects close by adherence of the septal tricuspid leaflet to the margins of the defect, less commonly by prolapse of an aortic cusp,24,31,44 and rarely by intrusion of a sinus of Valsalva aneurysm. Adherence of tricuspid leaflet tissue is seldom accompanied by tricuspid regurgitation, but there is a tendency for aneurysm formation when perimembranous defects are closed by the tricuspid valve (see Figures 17-4 and 17-31B).2,32,45–49 Ventricular septal aneurysms were described by Laennec in 1826 and are, as a rule, relatively small (see Figure 17-4). Exceptionally, however, septal aneurysms expand to considerable size, and a giant aneurysm of the membranous septum revealed itself as a mediastinal mass.50 A blind septal aneurysm occasionally represents a sui generis congenital malformation of the interventricular portion of the membranous septum rather than a sequel to spontaneous closure of a perimembranous defect.51 Familial congenital septal aneurysms have been reported, although rarely.52 With notable exceptions, aneurysms are well tolerated and have been discovered incidentally at necropsy in patients beyond the eighth decade. Complications of septal aneurysms include infective endocarditis, conduction disturbances, intraaneurysmal thrombosis with systemic embolism, aortic or tricuspid regurgitation, and obstruction to right ventricular outflow (see Figure 18-11).51 Blind congenital aneurysms may spontaneously perforate, establishing a left-to-right shunt into either the right ventricle or right atrium.51
The hemodynamic classification of ventricular septal defects is relatively straightforward because physiologic consequences depend essentially on the size of the defect and the pulmonary vascular resistance.53 These two variables change with time, and the physiologic and clinical manifestations change accordingly. A defect in the ventricular septum, even if spontaneously closed, has been postulated to constitute an abnormality that might adversely affect left ventricular systolic function.54 This postulation is relevant to impaired function or left ventricular failure in an occasional patient with a restrictive defect and a negligible shunt.54
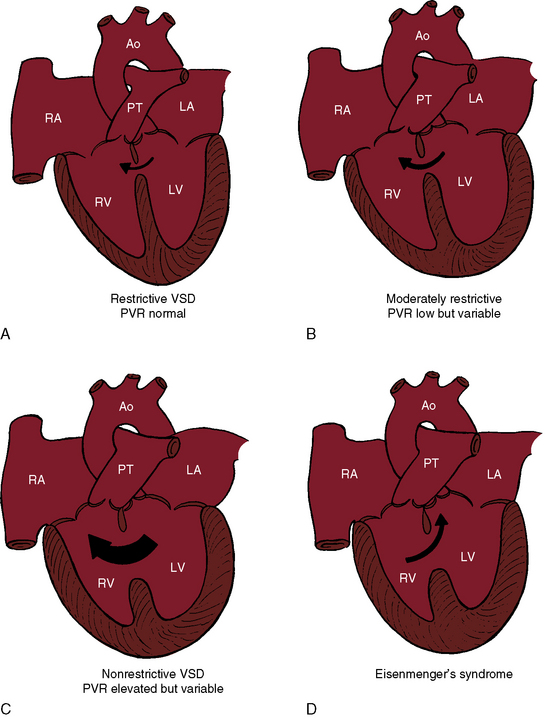
Ventricular septal defects fall into four anatomic/physiologic categories (Figure 17-5): (1) restrictive defects with normal right ventricular and pulmonary artery systolic pressure and normal pulmonary vascular resistance; (2) moderately restrictive defects with elevated right ventricular and pulmonary artery systolic pressures and low but variable pulmonary vascular resistance; (3) nonrestrictive defects with identical right ventricular and left ventricular systolic pressure and elevated but subsystemic pulmonary vascular resistance that is variable; and (4) nonrestrictive defects with identical right and left ventricular systolic pressures, suprasystemic pulmonary vascular resistance, and a right-to-left shunt—Eisenmenger’s syndrome.
Henri Roger’s account of the physiology of a restrictive ventricular septal defect with normal pulmonary vascular resistance still applies1:
Restrictive ventricular septal defects—maladie de Roger—cause little or no functional derangement because the shunt is small and the pressure and resistance in the pulmonary circulation are normal (see Figure 17-5A). A restrictive defect represents a site of obligatory resistance between the left and right ventricles, thus limiting (restricting) the magnitude of the left-to-right shunt (see previous) and precluding delivery of left ventricular systolic pressure into the right ventricle and pulmonary trunk. Shunt flow is systolic. Minor diastolic flow is generated by the normal differences in left and right ventricular distensibility and end diastolic pressure.
A moderately restrictive ventricular septal defect is characterized by right ventricular systolic pressure that is above normal but less than systemic and by low but variable pulmonary vascular resistance that rarely progresses. The left ventricle adapts to a moderate increase in volume, and the right ventricle adapts to a moderate increase in pressure. Instant-to-instant right-to-left shunting occurs because left ventricular systolic pressure falls more rapidly than right ventricular systolic pressure. However, left ventricular systolic pressure rises more rapidly than right ventricular systolic pressure, so the small right-to-left shunts are quantitatively returned to the right ventricle with the next cardiac cycle. An increase in end-diastolic pressure in the volume-loaded left ventricle results in diastolic shunting.55 Moderately restrictive defects in the trabecular muscular septum decrease in size during ventricular contraction and, in so doing, decrease the systolic shunt.56 With isotonic exercise, systemic vascular resistance falls while pulmonary vascular resistance changes little if at all,57 so a normal increment in systemic flow is achieved without a corresponding increase in left-to-right shunt.57
With a nonrestrictive ventricular septal defect, the right and left ventricles behave physiologically as a common chamber. Peak systemic systolic pressures are identical, so the volume and direction of flow through the defect depend on the relative resistances in the pulmonary and systemic circulations. A nonrestrictive defect with elevated but variable pulmonary vascular resistance (see Figure 17-5C) imposes an excessive volume load on the left ventricle and systemic afterload on the right ventricle. A persistently large left-to-right shunt culminates in depressed systolic function of the volume overloaded left ventricle.58 A rise in pulmonary vascular resistance results in a reciprocal fall in left-to-right shunt and a comparable reduction in volume overload of the left ventricle, but systolic pressure in the two ventricles necessarily remains identical. When pulmonary vascular resistance is suprasystemic, the architecture of the pulmonary vascular bed resembles primary pulmonary hypertension (see Chapter 14) and the left-to-right shunt is replaced by a right-to-left shunt, Eisenmenger’s syndrome (Figure 17-5D).37,59–65 Right ventricle function is analogous to the function of a fetal right ventricle that is equipped to cope with systemic vascular resistance.66
The vasoreactive immature pulmonary resistance vessels play a pivotal role in regulating pulmonary blood flow in neonates with a nonrestrictive ventricular septal defect. The physiologic fall in neonatal pulmonary vascular resistance is delayed because of the interplay between shunt size and pulmonary vasoreactivity. In infants with a moderately restrictive ventricular septal defect, two additional mechanisms serve to regulate the volume and direction of the shunt and the level of pulmonary arterial pressure. The most favorable mechanism is a decrease in size of the defect. A much less common mechanism is acquired obstruction to right ventricular outflow that reduces left ventricular volume overload and protects the pulmonary vascular bed (see Chapter 18).67 When and to what extent these regulatory mechanisms exert their influence determines the hemodynamic and clinical course in patients with moderately restrictive and nonrestrictive ventricular septal defects.37,62,68,69
History
Ventricular septal defects are found in a wide range of mammals and in birds with four-chambered hearts.4 The incidence in humans is unrelated to gender, race, maternal age, or birth order.4,70 Ventricular septal defects have been reported in 3.3% of first-degree relatives of index patients.71 One third of first-degree relatives of patients with congenital heart disease have ventricular septal defects.4 Between 30% and 60% of siblings of index patients have ventricular septal defects,72 and siblings of patients with ventricular septal defects have three times the incidence of ventricular septal defects compared to the general population.4 Ventricular septal defects have been reported in identical twins,73,74 but with a high frequency of discordance.4 A parent with a spontaneously closed ventricular septal defect can have offspring with a ventricular septal defect. Birth weights are low in about 18% of full-term infants with ventricular septal defects.75 Dysmaturity is in addition to and apart from occurrence of ventricular septal defects in preterm infants. Heterotrisomy is a significant factor in ventricular septal defects with Down syndrome.76
Restrictive ventricular septal defects come to light because a systolic murmur is detected at the first well-baby examination. A murmur that is present at birth is a feature of ventricular septal defect with a left ventricular-to-right atrial communication in which the shunt exists in utero and is therefore present at birth (see subsequent).77 Very small defects with trivial early systolic shunts escape detection because the early systolic murmurs are soft and are either undetected or mistaken for a normal or innocent murmur (see section Auscultation). Spontaneous closure accounts for the striking age-related disparity in the incidence of ventricular septal defects from birth to maturity.31,35,62 The patient is left with no shunt and a functionally normal heart that harbors a morphologic abnormality (see previous), especially if spontaneous closure is followed by a septal aneurysm (see Figure 17-4).32 The defect is necessarily missed if the physical examination is performed after spontaneous closure.
Infective endocarditis is a tangible risk in restrictive ventricular septal defects, but rarely occurs before eruption of the second teeth.62,67,78–80 Roger aptly stated, “Prevention of complications is by means of hygiene.”1 Infective endocarditis is usually located on the septal tricuspid leaflet at site of the jet impact. Muscular defects have a low incidence of infective endocarditis because the jet is dissipated within the right ventricular cavity without striking the septal tricuspid leaflet.15 Occasionally, the site of infective endocarditis is on an aneurysm of the ventricular septum,81 and rarely, vegetations at the base of the septal tricuspid leaflet cause perforation and a left ventricular-to-right atrial shunt, an acquired Gerbode defect.82
Longevity is likely to be normal, as Roger stated:
Several subjects whom I have been able to observe, I have attended for periods of five, twelve and fifteen years; these children have grown like others, not one has died prematurely. I have occasionally visited as physician a woman whose children I attended from early ages. She had always been in excellent health and had never complained of cardiac difficulties. On auscultation I was greatly surprised to hear a murmur. I asked her if physicians had ever found anything wrong with her heart, and she told me that Guersant the Elder (the famous pediatrician who was my first master in infantile pathology) had recognized in her a few days after birth a cardiac malformation. This woman has now passed her fiftieth year; her health continues to be perfect and she is the mother of four children. In spite of the existence of an uncomplicated defect in the interventricular septum, patients may attain or even surpass the average span of human life.1
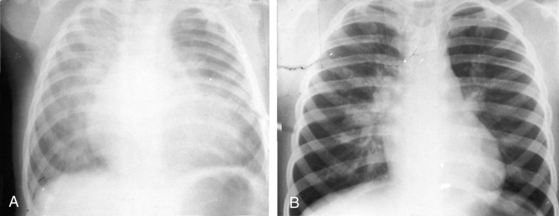
Moderately restrictive ventricular septal defects with low but variable pulmonary vascular resistance (Figure 17-5B) escape detection in the newborn nursery because the delayed fall in neonatal pulmonary vascular resistance delays the onset of the shunt and the onset of the murmur. Congestive heart failure within the first few months of life is in response to a large left-to-right shunt that is established after the fall in pulmonary vascular resistance. Parents report that their infant fatigues and coughs while feeding, sweats excessively, is restless when recumbent, and sleeps poorly. Parents may detect a thrill when they touch their infant’s chest and may detect a hyperactive precordium when holding the infant next to their chest. Symptomatic improvement is related to a spontaneous decrease in size of the defect, a favorable trend that may culminate in spontaneous closure (Figure 17-6; see previous).
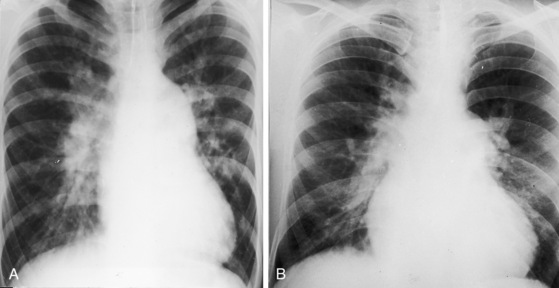
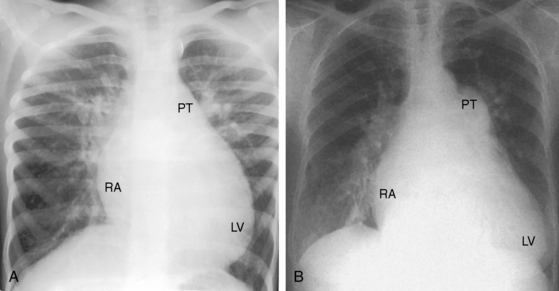
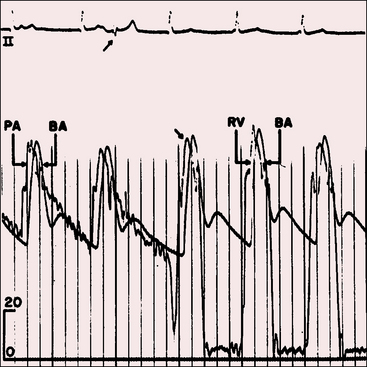
Patients with moderately restrictive defects and moderate left-to-right shunts tolerate isotonic exercise because the exercise-induced increment in systemic blood flow is achieved without a corresponding increase in shunt flow (see previous).57 However, persistence of a significant shunt incurs the risk of infective endocarditis, and protracted volume overload of the left ventricle incurs the risk of congestive heart failure.67 Only an occasional patient achieves adulthood (Figure 17-7), with one reaching age 65 years (Figure 17-8B) and another reaching age 79 years.83
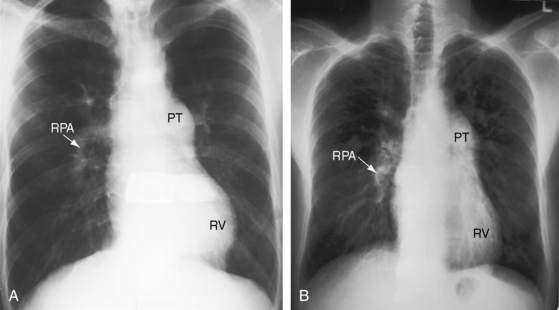
Nonrestrictive ventricular septal defects with elevated but variable pulmonary vascular resistance (see Figure 17-5C) present in infancy with congestive heart failure and with little prospect that the defect will decrease in size. Poor growth and development, labored breathing, frequent lower respiratory infections, difficulty feeding, and excessive diaphoresis are typical.37,67,84 Dyspnea and irritability are especially pronounced when the infant is supine and improve when the baby is held upright or is placed in an infant seat. Feeding patterns are also typical. A hungry infant awakens from a fretful sleep and feeds vigorously only to stop short of satisfaction because of dyspnea, falls asleep again, exhausted by the effort, and awakens with renewed hunger and repeats the frustrating cycle. Regulation of the large left-to-right shunt with amelioration of symptoms is almost always the result of a rise in pulmonary vascular resistance.25,38,62,68 A minority of these patients undergo relatively little fall in neonatal pulmonary vascular resistance, so the shunts are small and the early clinical course is deceptively benign. When pulmonary resistance exceeds systemic, the shunt is reversed, the patient is cyanotic,37,60,64 and the condition that Maude Abbott called Eisenmenger’s complex exists.85 Victor Eisenmenger published his account in 1897 in a paper entitled “Congenital Defects of the Ventricular Septum.”86,87 Paul Wood, in his landmark publication of 1958, introduced the term Eisenmenger’s syndrome (Figure 17-9), which he defined as pulmonary hypertension with reversed shunt.64 Wood quoted Eisenmenger:
Cyanotic congenital heart disease, represented here by Eisenmenger’s syndrome, is a multisystem systemic disorder that involves red cell mass, hemostasis, the central nervous system, bilirubin kinetics, the systemic vascular bed, the coronary circulation, the myocardium, uric acid clearance, the kidneys, respiration, the digits, the long bones, and gynecologic endocrinology.88,89 Morbidity and mortality take into account contemporary medical management of the multisystem disorders.88–90 Longevity is about one to two decades longer than in early reports.63,89,91 Right ventricular failure is uncommon66 unless acquired systemic hypertension imposes disproportionate afterload.
Erythrocytosis is an adaptive response to the decrease in tissue oxygenation—arterial hypoxemia—that stimulates renal release of erythropoietin, which in turn stimulates an increase in the number of red blood cells. Equilibrium conditions are established at elevated hematocrit levels. Erythrocytosis is a desirable adaptive response designed to offset the hypoxemic deficit in tissue oxygenation and does not incur a risk of stroke from cerebral arterial thrombosis irrespective of hematocrit level, iron stores, or cerebral symptoms of hyperviscosity.88 However, in cyanotic children younger than age 4 years, iron-deficient erythrocytosis incurs the risk of thrombosis of intracranial venous sinuses.88 The right-to-left shunts of cyanotic congenital heart disease are pathways for paradoxical emboli that cause transient ischemic attacks and strokes.
Hemostatic abnormalities have been recognized for more than 50 years in patients with cyanotic congenital heart disease.88 Platelet counts are in the low range of normal, and many clotting factor disorders have been identified, most recently acquired abnormalities of the von Willebrand’s factor.92 A mucocutaneous bleeding tendency is characterized by easy bruising, gingival bleeding, epistaxis, menorrhagia, and increased risk of traumatic bleeding. Pulmonary hemorrhage can be external (hemoptysis) or intrapulmonary, is the most serious type of bleeding, and varies from mild and occasional to copious, recurrent, massive, and fatal.64,88,93 Massive intrapulmonary hemorrhage is a common cause of sudden death in Eisenmenger’s syndrome.89 Victor Eisenmenger’s patient “died more or less suddenly … following a large hemoptysis.”64
Bilirubin is formed from the breakdown of heme, a process that is excessive in cyanotic congenital heart disease and that coincides with a substantial increase in the amount of unconjugated bilirubin in the bile.88 Calcium bilirubinate gallstones develop because unconjugated bilirubin is virtually water insoluble at physiologic pH.88
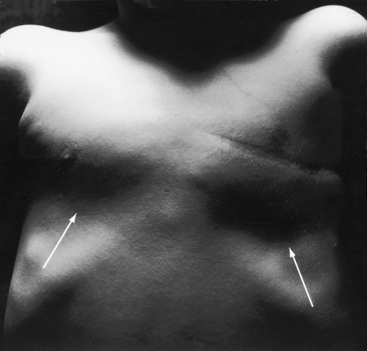
Systemic vascular dilation is the result of endothelial-derived nitric oxide and prostaglandins that are released in response to the increase in shear stress inherent in the erythrocytotic perfusate. Increased arteriolar dilation and tissue vascularity contribute to the bleeding tendency (Figure 17-10).88 Syncope can be caused by the heat-induced vasodilation of hot humid weather or by a hot shower or bath, especially when standing.
The extramural coronary arteries are dilated, elongated, and tortuous, even aneurysmal (coronary ectasia).88 Vasodilator nitric oxide and prostaglandins are released from coronary artery endothelium by the increased shear stress of erythrocytosis. Dilation of the coronary arteries is accompanied by an increase in extramural coronary artery blood flow that does not encroach on coronary vascular reserve because of remodeling of the myocardial microvascular circulation.94
Hyperuricemia results from decreased renal clearance and increased production of urate. Elevated plasma uric acid levels are common in cyanotic adults and are secondary to enhanced urate reabsorption that results from renal hypoperfusion reinforced by a high filtration fraction.88 Acute gouty arthritis is uncommon but not rare.
Renal involvement is represented by abnormalities of both structure and function.88,89 Decreased clearance of urate is a functional abnormality, but the resulting hyperuricemia in turn exerts little or no deleterious effect on renal function.88,89 Proteinuria is a functional abnormality caused by increased glomerular hydraulic pressure in response to the high viscosity of erythrocytotic blood entering the afferent glomerular arteriole in concert with obligatory ultrafiltration from afferent to afferent arterioles.
Two distinct structural (morphologic) glomerular abnormalities are found in cyanotic adults.89,95 The vascular abnormality is characterized by dilation of hilar arterioles and glomerular capillaries because of intraglomerular release of nitric oxide.95 The nonvascular abnormality is characterized by an increase in glomerular cellularity in response to platelet-derived growth factor and transforming growth factor beta in the cytoplasm of systemic venous megakaryocytes that are shunted into the systemic arterial circulation and lodge in glomerular tufts.95
Cardiorespiratory responses to isotonic exercise in cyanotic congenital heart disease significantly influence the dynamics of oxygen uptake and ventilation.88,96 Prolonged onset and recovery of oxygen uptake kinetics incur large oxygen deficits that result in hypoxemia even with low levels of exercise, which suggests that patients with right-to-left shunts rely heavily on anaerobic metabolism. Hyperventilation, which is subjectively perceived as dyspnea, is present at rest and increases excessively during exercise because augmentation of the right-to-left shunt induced by exercise is accompanied by an increase in systemic arterial carbon dioxide and a decrease in pH that stimulate the respiratory center and carotid bodies.88,96
Clubbing of the digits and hypertrophic osteoarthropathy share a common pathogenesis.88 Systemic venous megakaryocytes are shunted into the systemic arterial circulation (see previous) and impact in the digits and subperiostium. Platelet-derived growth factor and transforming growth factor beta are released from megakaryocytic cytoplasm and promote cell proliferation, protein synthesis, connective tissue formation, and deposition of extracellular matrix that are responsible for clubbing and hypertrophic osteoarthropathy.88
Pregnancy poses an excessive risk for women with Eisenmenger’s syndrome.87 The maternal mortality rate exceeds 50%, and there is an independent excessive risk of fetal wastage because of uterine hypoxemia associated with cyanosis.62,89,97,98
Sudden death often results from massive intrapulmonary hemorrhage,89 less commonly from rupture (dissection) of a dilated hypertensive pulmonary trunk,65,99 and rarely from ventricular tachycardia. A cerebral abscess in early life can result in a seizure disorder in adulthood, or the abscess can originate in adulthood.64,88
Physical Appearance
The abnormalities in physical appearance are the result of cyanosis caused by the reversed shunt in Eisenmenger’s syndrome, the catabolic effects of congestive heart failure in infants (poor growth and development, frailty, and cachexia),62 and Harrison’s grooves caused by the thoracic retractions of chronic dyspnea (Figure 17-11). Growth and development are also influenced by intrauterine and genetic factors and by low birth weight.75 Infants and young patients with nonrestrictive ventricular septal defects and balanced shunts may become cyanotic only after crying or exercise.100 Doubly committed subarterial ventricular septal defects are more common among Asians (see previous).17 The physical appearance of trisomy 18 Down syndrome (see Figures 19-5 and 20-11) coincides with the presence of an inlet ventricular septal defect (see Figures 15-90 and 15-91).101–104 In addition, a relationship exists between ventricular septal defect and physical appearance in trisomy 13; trisomy 8 and 9 mosaic; rare aberrations, such as 5p (cri du chat) and 13a- and 18q-105; Holt-Oram syndrome29; Cornelia de Lange’s syndrome72; Klippel-Feil syndrome54; cardiofacial syndrome106; and fetal alcohol syndrome.107
Arterial Pulse
Moderately restrictive defects with relatively low pulmonary vascular resistance are associated with a brisk arterial pulse because of vigorous ejection from a normal volume-loaded left ventricle. A diminished arterial pulse with pulsus alternans accompanies nonrestrictive defects with large left-to-right shunts and congestive heart failure. The arterial pulse in Eisenmenger’s syndrome is normal because systemic stroke volume is maintained (Figure 17-12).64
Jugular Venous Pulse
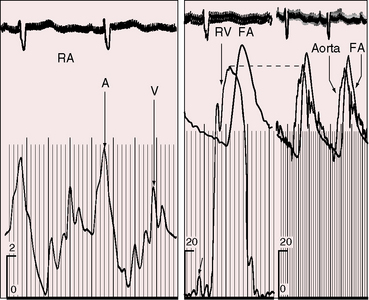
Moderately restrictive and nonrestrictive ventricular septal defects with congestive heart failure are accompanied by an elevated mean jugular venous pressure and an increase in A and V waves. However, the jugular venous pulse in Eisenmenger’s syndrome is normal or nearly so, with a small dominant A wave (Figure 17-13). Large A waves are exceptional because right ventricular systolic pressure does not exceed systemic. Systemic afterload exists from birth, so the right ventricle requires little extra help from its atrium.
Precordial Movement and Palpation
Roger stated that when a ventricular septal defect is restrictive, “it coincides with no other sign of organic disease with the exception of the harsh thrill which accompanies it.”1 The thrill is maximal in the third or fourth left intercostal space at the left sternal border. A subarterial ventricular septal defect directs its shunt into the pulmonary trunk, so the accompanying thrill is maximal in the second or first and second left intercostal space with radiation upward, to the left, and into the neck.108
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree