(1)
Department of General surgery, Fuwai Hospital, Beijing, China
This chapter focuses primarily on the primary ventricular septal defect (VSD). The VSD that occurs as a component of other major cardiac malformations is discussed in other chapters.
13.1 Classification of VSDs
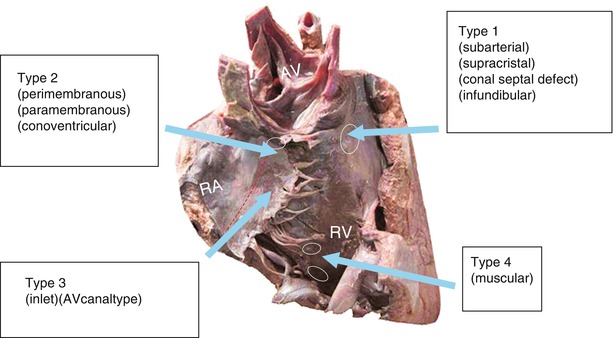
Several systems are used to classify VSDs, and the differences among them are not significant. In 2000, Jacobs introduced the consensus of the Congenital Heart Surgery Nomenclature and Database Project (Box 3.1), which divides VSDs into four subtypes as follows (Fig. 13.1):
Type I subarterial VSD
Type II perimembranous VSD
Type III AV-type or inlet-type VSD
Type IV muscular VSD (Fig. 13.5)
In China, no consensus has been reached for classifying VSDs. Box 13.1 lists the classification system used most frequently in Fuwai Hospital, Beijing.
Box 13.1 Classification of VSDs
Subtype | Definition | |
|---|---|---|
Infundibular VSD | Subarterial | The upper margin of the defect consists of part of the pulmonary and aortic annuli |
Intracristal | The defect is surrounded by muscular tissue; the infundibular septum is separated from the tricuspid annulus by a band of muscle | |
Membranous VSD | Simple membranous | The margins of the defect are fibrous tissue |
Perimembranous VSD | Infracristal | The defect involves membranous septum and partial supraventricular crest, located anterior to the papillary muscle of the conus artery |
Juxtatricuspid | The defect involves membranous septum and partial sinus septum, located posterior to the papillary muscle of the conus artery | |
AV canal type | A large defect exists in the sinus septum | |
Muscular VSD | The defect is in the sinus septum and the trabecular septum, with entire muscular borders |
A simple membranous VSD (Figs. 13.4 and 13.3) can be barely distinguishable from an infracristal VSD. Generally, the former is small and bordered by intact white fibrous tissue, whereas the infracristal VSD and the juxtatricuspid VSD are relatively larger. Both simple membranous and infracristal VSDs are quite common occurrences, whereas a muscular VSD is rarely seen.
Because the hypoplastic membranous portion of the atrioventricular septum, also known as the left ventricular–right atrial defect, is relevant to the development of the endocardial cushion and has no agreed-upon classification, it can also be classified as a VSD.
13.2 Anatomical Key Points of VSDs
1.
Small VSD (Figs. 13.14, 13.6, and 13.11) is a small defect bordered by white fibrous tissue with a diameter less than 5 mm. The predilection site in the embryonic period is the commissural line of the ventricular septum, especially the inferior edge of the supraventricular crest (equivalent to the commissural line of the anterior and posterior ventricular septum in the embryonic period) and the bulbar ridge (equivalent to the commissural line of the conus artery ridge in the embryonic period). These small VSDs can be sutured directly without patch due to their strong edge. In many cases of small single membranous VSDs, the defects are bordered by the TV and fibrous tissue. The external aperture of the defect seems to be surrounded by a fibrous canal when it is viewed from the RV. The suture line should be placed on the base of the VSD, rather than on the aperture of the canal, to avoid having a loose stitch. Sometimes a fibrous stripe is found across the defect. If it is mistaken for the edge of the defect, a residual defect may be left.
2.
Large subarterial VSD (Figs. 13.12, 13.15, and 13.16) is tear shaped rather than circular and has no muscular upper margin. Parts of the aortic and pulmonary annuli form the upper margin. The front of the right coronary cusp can be seen through the defect. The AV is easily injured, and the conduction system remains at a distance from the defect. The AV should be protected during the operation.
3.
Above the large infracristal VSD (Figs. 13.8, 13.9, and 13.2) is the right coronary cusp of the AV, and the posterior edge of the defect, which is either fibrous or muscular, extends to the tricuspid annulus. A conduction bundle runs along the posterior-inferior edge of the defect. The papillary muscle of the conus artery begins posterior to the defect, and the supraventricular crest lies anterior to the defect. The defect usually is large and should be repaired with a patch rather than direct suture. The conduction system should be protected when repairing the posterior inferior edge of defect.
4.
The defect beneath the septal leaflet of the TV and the AV canal type of VSD (Figs. 13.13, 13.19, 13.20, 13.7, and 13.24) are usually long and slender, located below the septal leaflet of the TV. The tricuspid annulus forms the upper margin of the defect and is located posterior to the papillary muscle of the conus artery. The anterior margin remains at a distance from the AV. Most of the defect can be obscured by the septal leaflet of the TV.
13.3 Surgical Technique: Difficulties and Risks
The purpose of VSD repair is to close the defect and to eliminate left-to-right shunt. Transcatheter closure of ventricular defects is limited, so open-heart surgery is the main method. Any fault or risk can appear during operation, including:
1.
A residual, fistula, small membranous defect may be divided into two holes by a fibrous stripe or be surrounded by the fibrous canal below the TV. The hole may be mistaken for the entire defect during operation, resulting in residual shunting and murmur.
In a large VSD repair or an infant VSD repair with patch, the suture line may be too tight and shallow, cutting the muscular edge and leading to residual shunting. Residual shunting also may appear if continuous stitches are not tightened (Figs. 13.21, 13.22, 13.23, 13.17, and 13.18).
2.
AV injury leads to aortic insufficiency. In the case of a large membranous VSD, especially a subarterial VSD, the AV may be injured during surgery, resulting in avulsion and perforation of the cusp. Traction from the suture line may cause deformation of the valve. When these situations arise, reoperation should be performed decisively to repair the injured AV (Fig. 13.10).
3.
Complete atrioventricular block may occur. An atrioventricular bundle usually runs along the posteroinferior edge of a membranous VSD (including an infracristal VSD ), close to the right ventricular side. Sutures that are too deep may injure the perforating branch or the left and right bundle branch of the bundle of His, resulting in heart block. Violent traction of local tissue may cause temporary or permanent atrioventricular block (Fig. 13.25).
4.
Traction of the TV leads to tricuspid insufficiency. A large membranous and perimembranous VSD may be obscured by the chordae of the TV and the papillary muscle of the conus artery. Part of the leaflet or the chordae may be sutured onto the patch so that the TV closure is disturbed, leading to tricuspid insufficiency.
13.4 Specimen Demonstrations
Location of VSDs




Type 1 subarterial/supracristal/conal septal defect/infundibular
Type 2 perimembranous/paramembranous/conoventricular
Type 3 inlet/AV canal type
Type 4 muscular

Fig. 13.1
International classification of VSD in contrast to others. The figure demonstrates four common types of VSDs viewed from the RV of the normal heart

Fig. 13.2
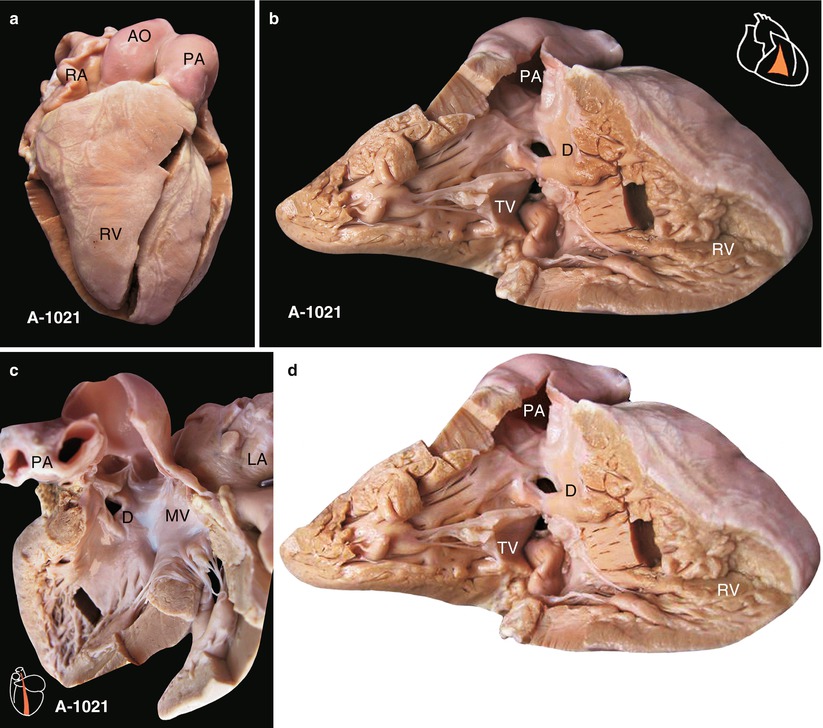
(a) Specimen 1021. Small intracristal VSD with normal heart contour. (b) Specimen 1021. Right ventricle-pulmonary artery cut open. The anterior wall of the RV is elevated to show the TV, pulmonary artery, and VSD. The defect has intact muscular margins and infundibular muscular tissue that can be seen between the posterior inferior border of the defect and the tricuspid leaflet, so the defect is inside the infundibular septum, representing precisely a small intracristal VSD. (c) Specimen 1021. VSD viewed from left ventricle. Defect is away from the membranous septal, so it is not a membranous VSD. The figure also shows the fibrous continuity between the AV and the anterior leaflet of the MV. LA left atrium, MV mitral valve, PA pulmonary artery. (d) Specimen 1021. Surgical interpretation for a small intracristal VSD. (1) Intracristal VSD can be repaired with a patch or direct suture, avoiding injury to conducting tissue because it is bordered by muscle. (2) The VSD occlude is easily released through the TV

Fig. 13.3
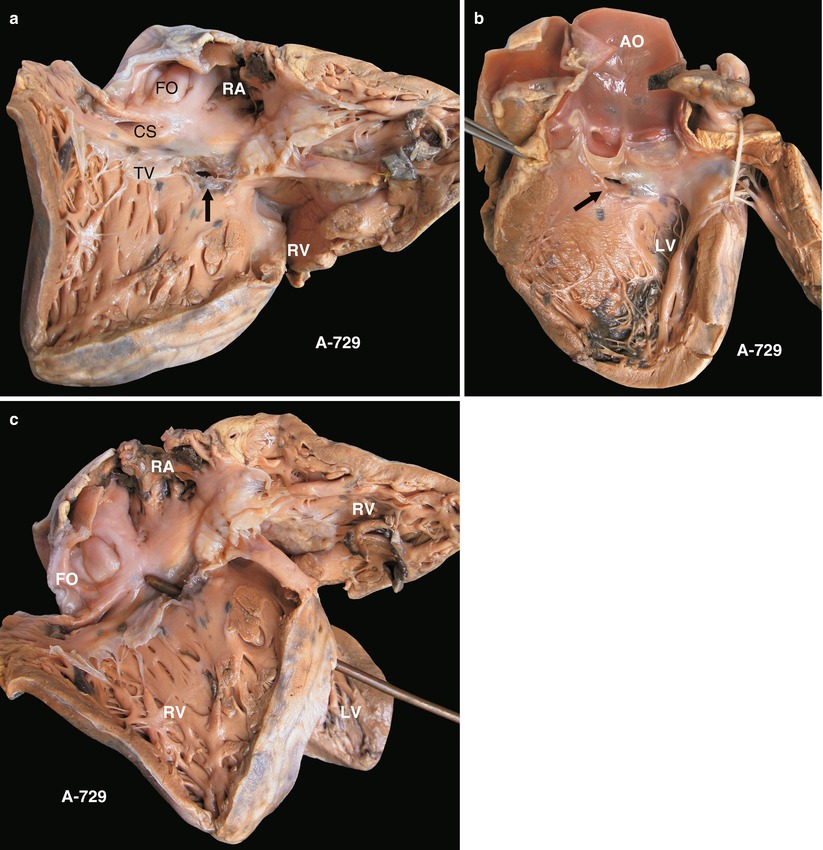
(a) Specimen 729. Membranous VSD. The defect is located at the anteroseptal commissure of the TV (arrow). CS coronary sinus, FO fossa ovale, TV tricuspid valve. (b) Specimen 729. Membranous VSD viewed from the left ventricle. As the arrow indicates, the defect was beneath the right aortic cusp and the noncoronary cusp. (c) Specimen 729. The probe penetrated the membranous VSD from LV to RV. LV left ventricle; RV right ventricle

Fig. 13.4
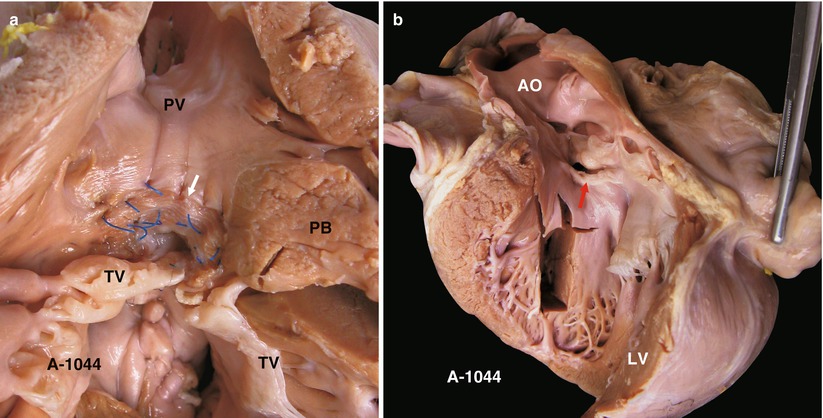
(a) Specimen 1044. Small membranous VSD viewed from the right ventricle. White arrow indicates the patch. Surgical suture of the VSD has been partly removed. PB parietal band, TV tricuspid valve. (b) Specimen 1044. VSD (arrow) viewed from left ventricle

Fig. 13.5
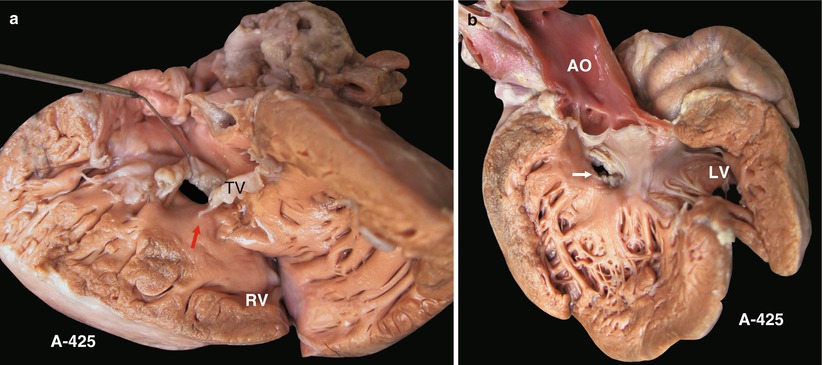
(a) Specimen 425. VSD beneath septal leaflet of tricuspid valve. The septal leaflet of the TV is elevated to demonstrate the VSD. The upper margin is attached to the edge of the septal leaflet. The TV entrance is obscured. The arrow indicates papillary muscle of the conus artery. TV tricuspid valve. (b) Specimen 425. Viewed from left ventricle (arrow). VSD is located beneath the septal leaflet of the TV and is away from the AV. AO ascending aorta, LV left ventricle

Fig. 13.6
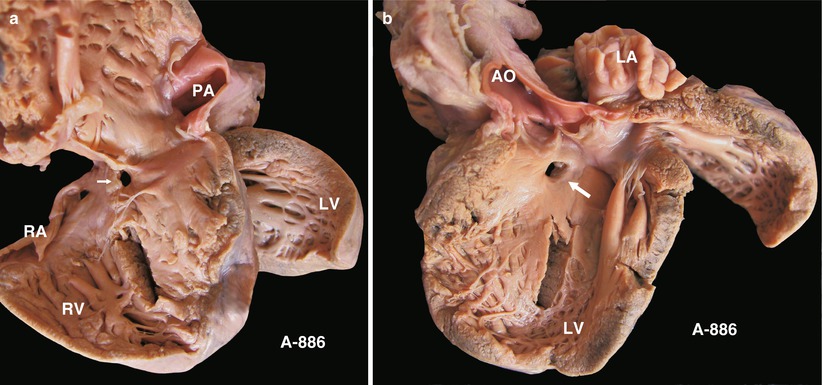
(a) Specimen 886. Typical small membranous VSD. The defect is near the TV orifice and can be seen clearly via a right atrial incision in the operation. (b) Specimen 886. Defect viewed from left ventricle. Note the spatial relationship between the VSD and the AV
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


