Ventricular Assist Devices for Infants and Children: State of the Art and Future
Iki Adachi
Charles D. Fraser Jr.
INTRODUCTION
The last decade has witnessed significant advancements in ventricular assist devices (VADs) for the adult population. Continued refinement of device technology as well as clinical management has led to improved outcomes of VAD support, resulting in a rapid increase in the number of patients supported with VADs (1). In stark contrast to the adult population, options for mechanical circulatory support (MCS) in children, particularly infants and small children, are limited by a paucity of devices designed for pediatric use (2). In fact, extracorporeal membrane oxygenation (ECMO) used to be the only MCS option in this group of patients. This frustrating reality, however, is starting to change. Owing to widespread acceptance of the Berlin Heart EXCOR (Berlin Heart, The Woodland, TX), which is equipped with a variety of pump and cannula sizes, even infants can now benefit from longterm VAD support. In addition, the Infant Jarvik 2000 (Jarvik Heart Inc., New York, NY), an implantable, continuous-flow VAD for small children, will be analyzed for safety and efficacy in its first randomized trial (3). Finally, the era of pediatric MCS has begun. In this chapter, we will review VAD devices available for the pediatric population.
RECENT DRAMATIC CHANGES IN HEART FAILURE MANAGEMENT
In recent years, VADs have become an integral component of the standard of care provided to adults with end-stage heart failure. The most significant change to impact patient management strategy is the emergence of implantable, continuous-flow VADs such as the HeartMate II (Thoratec Corporation, Pleasanton, CA) and the HeartWare HVAD (HeartWare Inc., Framingham, MA). The efficiency of these devices, coupled with low-morbidity profiles, has contributed to a change in indications for device placement. Early utilization of such a device is now considered a reasonable option in preference to escalating medical management. Nowadays, VADs are being used not only for bridging the patient to heart transplantation (bridge to transplant, BTT) but also as destination therapy (DT), which is currently offered only to those candidates deemed unsuitable for transplantation. In fact, the number of patients who undergo VAD placement for DT has increased, now accounting for about 41% of all adult VAD implantations (1). It is suggested that with continuing evolution of VAD therapy, the medical community is approaching the point where VADs may be considered the primary alternative to cardiac transplantation (4,5).
Although the pediatric population has not yet experienced this shift in the MCS paradigm, there has been a growing interest in pediatric MCS in recent years for the following reasons: First, the number of children with endstage heart failure far exceeds the availability of suitable pediatric cardiac donors. The number of pediatric heart transplants worldwide has been stagnant at approximately 300 to 400 per year (6,7). By analyzing 15 million pediatric hospitalizations using the Healthcare Cost and Utilization Project Kids Inpatient Database, Rossano et al. have shown that heart failure-related hospitalizations occur in 11,000 to 14,000 children annually in the United States, with an overall mortality of 7% (8). It is important to note that admissions did not greatly increase over the 10-year study period (1997-2006), but the total mean length of stay increased from 13.8 to 19.4 days. This data possibly indicates a temporal increase in the proportion of sicker children. Secondly, an increment in the number of children listed for heart transplantation would support this view, which has inevitably resulted in prolongation of wait times on the heart transplant list in the setting of unchanged organ supply (6). While ECMO has traditionally been used for bridging the patient to transplantation, the outcome of such an intervention is generally poor. Inherently temporary, ECMO support cannot be instituted indefinitely until a suitable organ is found. This limitation has gained greater significance in recent years where waitlist duration has been steadily increasing. Using the ELSO Registry, Almond and associates (9) found that a third of pediatric heart transplant recipients on ECMO support as a BTT die during the same admission. Five- and ten-year posttransplantation survival is also worse in patients on ECMO prior to transplant than in patients who were on a VAD or not on MCS (10).
INDICATIONS FOR VENTRICULAR ASSIST DEVICES
At present, there is no universal consensus with regard to indications for instituting VAD support. In general, VAD therapy for any patient is considered when the proposed benefits of VAD outweigh the risks. Traditionally, VAD support is indicated when severity of heart failure extends beyond the capability of maximal medical management. As with all interventions, a practitioner’s individual threshold in deciding whether to provide or withhold a therapy is influenced by the degree of confidence in that modality to consistently produce positive outcomes. Since the risk-to-benefit ratio is largely determined by the patient’s clinical status, institutional experience, and type of devices available, the decision needs to be made on a case-by-case basis by an experienced multidisciplinary team. The Fifth Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) report found that preoperative clinical status of a VAD candidate has an appreciable impact on postimplant outcomes (5). There are ongoing discussions about extending partial VAD support to include patients with INTERMACS profile 4 to 7 (1 being worst, 7 being best) who may derive some benefit (11,12). The trend of early VAD institution has so far resulted in considerably improved outcomes after VAD implantation (1).
Pediatric physicians, contrary to adult physicians, tend to be more judicious when evaluating indications for VAD use in children. Globally, most pediatric heart centers have less experience with such devices as compared with adult centers. This is particularly true for small children, who can only be supported with pulsatile, extracorporeal VADs (e.g., Berlin Heart EXCOR) that are associated with significantly higher risk profiles than implantable, continuous-flow devices (13). Devices such as these present a challenge to clinicians with respect to determining the optimum timeframe within which to commence therapy, as waiting too long to implant the EXCOR sharply increases the risk of mortality, while initiating EXCOR support too soon increases the risk of device-related morbidities. The ideal timeframe for implanting the device is, unfortunately, debatable and dependent on several variables (14). Further complicating matters, there are several contraindications to VAD support in children including, but not limited to, extreme prematurity, low body weight (<2.0 kg), preexisting neurologic injury, and congenital anomalies or major chromosomal aberrations with poor prognosis. In addition, multisystem end-organ injury presents a relative contraindication, but does not necessarily preclude patients from consideration of VAD support if end-organ recovery is predicted with improved cardiac output. Again, individual assessment of patient risk profile is of critical importance.
At Texas Children’s Hospital (TCH), the application of the Berlin Heart EXCOR is limited to critically ill children with cardiogenic shock or end-organ injury (INTERMACS profile 1 or 2) (15). The reality is that the vast majority of these patients who undergo Berlin EXCOR implantation are already on ECMO support or mechanically ventilated. In older children who can accommodate an adult-sized implantable device (e.g., HeartMate II or HeartWare HVAD), TCH initiates VAD support sooner, typically at INTERMACS profile 2 or 3. Lower risk profiles of these implantable devices, compared to an extracorporeal pulsatile VAD, justify such an early initiation. Certain clinical circumstances have to be carefully considered before VADs are implanted in children: Congenitally malformed hearts have anatomic variations, such as abnormal size and location of the aorta or unusual location or shape of the ventricle, that pose technical challenges, specifically with regards to placement of inflow and outflow cannulas. In addition, previous surgical palliation may jeopardize the provision of MCS due to further anatomic and circulatory physiologic disorder, such as systemic-to-pulmonary artery shunts or disconnected vena cava and pulmonary artery after the Glenn or Fontan operations. Furthermore, unique pathophysiologic features of heart failure must be taken into account when considering VAD support for patients with single-ventricle physiology. For instance, device selection for the failing Fontan circulation requires careful assessment of the etiology, which can be multifactorial. If systemic ventricular dysfunction is the primary impediment to the Fontan circulation, placement of a VAD to support the failing systemic ventricle would suffice (16). A total artificial heart may be necessary, however, when the Fontan circulation is restricted at multiple levels (e.g., ventricular dysfunction, atrioventricular valve insufficiency, and high pulmonary vascular resistance) (17). In addition, psychosocial and family counseling should be offered, particularly if the possibility of home discharge with an implantable VAD arises, to optimize outpatient management.
CHOOSING THE MOST APPROPRIATE MODE OF SUPPORT
ECMO versus Ventricular Assist Device
ECMO is the initial support of choice for patients unable to be weaned off cardiopulmonary bypass (CPB) or failure of return of spontaneous circulation after cardiac arrest (extracorporeal cardiopulmonary resuscitation, ECPR) (18). Beyond this, there are two major elements that factor in to deciding what type of MCS is required. These are the anticipated duration of support and whether the support should be univentricular or biventricular. The anticipated duration of support is largely dependent on the etiology of the heart failure, and whether it is acute (e.g., viral myocarditis), or chronic (e.g., dilated cardiomyopathy). Short-term support is offered to patients with acute-type or unknown etiologies, or if transplant candidacy is uncertain. The objective is hemodynamic optimization, thereby allowing for further investigation to assist in decision making (bridge-to-decision). If, at this point, longterm support is deemed necessary in order to bridge to transplant, or until possible cardiac recovery, temporary MCS can
be transitioned to long-term VAD (bridge-to-bridge). The second determinant is deciding whether left ventricular support (LVAD), right ventricular support (RVAD), or both, biventricular support (BiVAD), are needed. Table 32.1 lists the modes of support and the devices currently available for use in the pediatric population.
be transitioned to long-term VAD (bridge-to-bridge). The second determinant is deciding whether left ventricular support (LVAD), right ventricular support (RVAD), or both, biventricular support (BiVAD), are needed. Table 32.1 lists the modes of support and the devices currently available for use in the pediatric population.
TABLE 32.1. Modes of support and devices currently available for use in children | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||
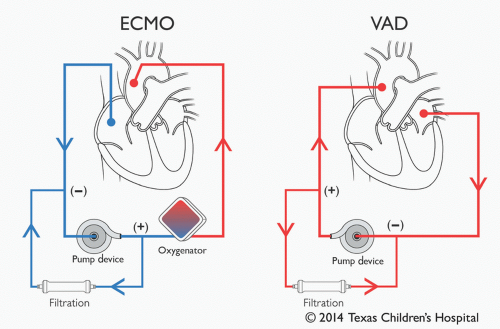
Since acutely decompensated heart failure often results in pulmonary dysfunction, affected patients may need additional pulmonary support by way of ECMO. Although many pediatric heart centers around the world promote the use of ECMO as the first-line MCS strategy, intact pulmonary function notwithstanding, TCH reserves ECMO support for when pulmonary support is clearly needed. Due to the presence of an oxygenator, we believe that application of ECMO is appropriate and should be limited to patients in whom pulmonary support is needed. ECPR, as stated above, is a typical situation necessitating its use. Other potential applications include severe pulmonary edema secondary to ventricular dysfunction, pulmonary hypertension, hemodynamic instability due to septic shock, or all situations where pulmonary function is or may become threatened (19,20). For purely circulatory support, the use of a VAD is favored. As ECMO is described in detail in Chapter 31, the rest of this chapter will be dedicated to discussing VADs.
Short-Term Ventricular Assist Device
It warrants repeating, in our opinion, that short-term VAD support is the quintessential MCS option for acute causes of heart failure where recovery of cardiac function is expected in a relatively short period of time (i.e., 2 weeks or less). A compelling reason for this preference over ECMO is direct decompression of the left heart, providing both the myocardium and the lungs with the best chance for recovery. Comparatively, ECMO requires placing a venous inflow cannula in the right side of the heart which has indirect, and hence limited, effects on the decompression of a failing left ventricle. In patients with an intact atrial septum, a left atrial cannula may need to be placed to adequately decompress the left ventricle and lower left atrial pressures.
Central Cannulation
The basic components of an extracorporeal short-term VAD system include a pump head and console, circuit tubing, and inflow and outflow cannulas (Fig. 32.1). Several centers use a centrifugal pump, such as the CentriMag (flow range up to 10 L/min) or PediMag (flow range 0.4-1.7 L/min) (Thoratec, Pleasanton, CA; Fig. 32.2A) or Rotaflow (Maquet, Germany; Fig. 32.2B). Although LV apical cannulation is an acceptable alternative (21), our preferred approach is placing the inflow cannula in the left atrium, either from Waterston’s groove or the left atrial appendage, in an effort to avoid further aggravating an already damaged left ventricle. We have found that left atrial cannulation provides very stable inflow function. We have fortunately not experienced the serious potential complication of left ventricular thrombus formation with left atrial cannulation. Although some would consider sternotomy a significant disadvantage of short-term VAD support as compared to ECMO, this central access provides unparalleled advantages of employing larger cannulas for direct drainage of the left heart that will result in superior pulmonary protection. This is particularly useful in conditions with increased cardiac return (e.g., systemic-to-pulmonary artery collaterals) that is often encountered in the pediatric population. The same degree of decompression cannot be achieved with peripheral ECMO where a smaller-sized inflow cannula is inserted into the right-sided cardiac chamber. The outflow cannula is typically inserted into the ascending aorta. If there is a possibility that the short-term VAD may need to be converted to a long-term VAD (bridge-tobridge), the outflow cannula for the short-term VAD should be inserted in the distal ascending aorta or even in the proximal arch so that there is sufficient space in the proximal ascending aorta for a long-term VAD outflow cannula.
Peripheral Cannulation
Peripheral cannulation is an alternative approach to central cannulation via sternotomy for short-term VAD support. The use of peripheral cannulation requires an inflow venous cannula to be inserted via the femoral vein and advanced into the left atrium across the interatrial septum (22). A distinct advantage of peripheral cannulation is the ability to establish VAD support quickly by negating sternal access, which is particularly helpful in cases of hemodynamically unstable children who have had a previous sternotomy.
The TandemHeart (CardiacAssist Inc., Pittsburgh, PA; Fig. 32.3) is one currently available short-term VAD system allowing peripheral cannulation. This system uses a centrifugal hydrodynamic pump and a 21Fr transseptal venous cannula (62 or 72 cm in length). Due to the relatively large size of this venous cannula, its application in the pediatric population is limited to older children and adolescents (approximately >40 kg in weight). Another potential problem with transseptal venous cannulation is the risk of cannula dislodgment. The patient will become severely hypoxic if the cannula dislodges into the right atrium. This risk is increased in children where the left atrium is much smaller than in adults. Arterial cannulation is with a 17Fr cannula placed in the femoral artery.
Another minimally invasive, catheter-based VAD that can also be placed percutaneously is the Impella (Abiomed, Inc., Danvers, MA; Fig. 32.4). This device has an axial flow pump and is available in a variety of sizes. The smallest pump of this family, the Impella 2.5 (specifications: flow rate up to 2.5 L/min, 9Fr catheter, 12Fr pump motor) has been used successfully in the pediatric population (23). Although cannulation is usually femoral, insertion via the right common carotid artery has been reported in an animal model (24). The company has a pediatric initiative and is currently developing a pediatric-specific line of products (25).
Initiation of Short-Term VAD Support
Once LVAD support has been initiated, inotropic support should be reduced appropriately, but not discontinued because the right ventricle requires pharmacologic support. Inhaled nitric oxide can be used in an attempt to reduce pulmonary vascular resistance. Initiation of vasodilator drugs may be necessary to modulate systemic vascular resistance and control hypertension. Once the ideal VAD flow is achieved, and systemic arterial pressure optimized, adequate systemic perfusion is confirmed by clinical and laboratory parameters. Adjustment of ventilator settings to maintain low pulmonary vascular resistance is essential to maximizing right-sided cardiac output, which will ultimately determine the filling status of the LVAD pump. Temperature control can be problematic, particularly in small children with an open chest. Unlike an ECMO circuit, the short-term VAD circuit does not have a heat exchanger. In such circumstances, an overhead radiant infant heater or an external convective warmer may be used. Serial assessment of cardiac function is used as a guide to weaning
mechanical support. In our experience with the use of shortterm VADs, the majority of patients with acute etiologies (17 out of 20, 85%) were successfully weaned off VAD support (26). Although all patients with acute myocarditis (n



mechanical support. In our experience with the use of shortterm VADs, the majority of patients with acute etiologies (17 out of 20, 85%) were successfully weaned off VAD support (26). Although all patients with acute myocarditis (n
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree