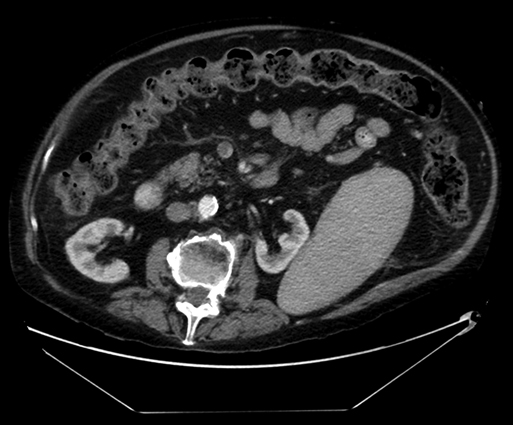
Sherry Cavanaugh, Ankur Gupta, Marc Friedman and Bruce L. Gewertz Computed tomography (CT) scans with venous delay is the most commonly used diagnostic modality for mesenteric vein thrombosis and compares favorably in sensitivity and specificity to selective mesenteric arteriogram with venous runoff (Figure 1). In most series, the sensitivity of CT in detecting acute thrombus of medium-sized or large vessels is 90% or greater. The diagnosis is made by noting central lucencies in the major veins (evident in up to 75% of cases) and correlating such findings with evidence of ascites, bowel or mesenteric edema (stranding), and inflammation. Water can be given orally to enhance the evaluation of the mucosa. Transmural infarction is suspected when a lack of bowel wall enhancement is seen. Other imaging modalities such magnetic resonance imaging (MRI) and ultrasonography are occasionally helpful in selected cases but do not supplant the utility of CT.
Venous Thrombosis Within the Splanchnic Circulation
Mesenteric Vein Thrombosis
Epidemiology
Diagnosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine