43 Venous Intervention
 The Venous System: Basic Histology and Physiology
The Venous System: Basic Histology and Physiology
Chronic Venous Disease
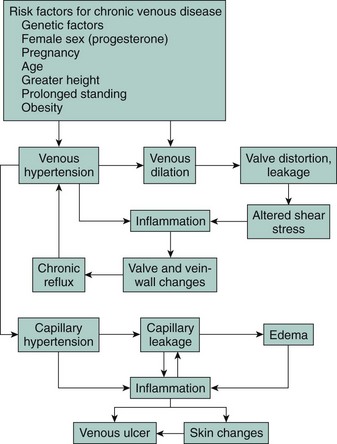
Chronic venous insufficiency is a significant problem in the United States, affecting as much as a quarter of the population. Venous valve incompetence is central to the underlying venous hypertension that appears to underlie most or all signs of chronic venous disease. Chronic venous disease affects a younger segment of the population; the resulting morbidity of edema, leg pain, and ulceration may then lead to lifestyle alterations, loss of work, and frequent hospitalizations. The prevalence of venous ulcerations is not restricted to the elderly but certainly increases with age.1 It has been estimated that venous ulcers have had a major negative economic impact, with the loss of approximately 2 million working days and treatment costs of approximately $3 billion per year in the United States.2 The chief clinical manifestations of chronic venous disease are aching, leg pain, heaviness, a sensation of swelling, itching, cramps, and restless legs. Chronic venous disease can be graded according to the descriptive clinical, etiological, anatomical, and pathophysiological (CEAP) classification, which provides an orderly framework for communication and decision making3 (Table 43-1). The pathophysiology of chronic venous disease in regard to its clinical expression has been well described, involving venous valve incompetence, structural changes in the vein wall (manifest as hypertrophy), and the role of elevated venous pressure and shear stress.4 There has been much newer work in understanding the pathophysiology of the skin changes of chronic venous disease. These studies have emerged to validate that chronic inflammation has a key role in the skin changes of chronic venous disease. Support for the role of chronic inflammation in chronic venous disease has come to be known as the microvascular leukocyte-trapping hypothesis, where elegant studies have shown elevated numbers of macrophages, T lymphocytes, and mast cells in skin biopsy specimens from lower limbs affected by chronic venous disease.5 The chronic inflammatory state in patients with chronic venous disease is related to the skin changes typical of the condition.4 Increased expression and activity of metalloproteinase (MMP, especially MMP2) has been reported in lipodermatosclerosis,6 venous leg ulcers,7 and wound fluid from nonhealing ulcers.8 The treatment of chronic venous disease is beyond the scope of this chapter but would be aimed at preventing venous hypertension, venous reflux, and chronic inflammation (Fig. 43-1). Compression stockings and devices are certainly the mainstay of controlling venous hypertension. New endovenous ablation procedures utilizing laser energy or radiofrequency are available to treat venous reflux. Clinical research is now being actively pursued seeking pharmacotherapy to alleviate the chronic inflammatory state of chronic venous disease, particularly targeting the interaction of leukocytes and endothelial cells.
TABLE 43-1 Revised Clinical Classification of Chronic Venous Disease of the Leg*
| Class | Definition | Comments |
|---|---|---|
| C0 | No visible or palpable signs of venous disease | |
| C2 | Telangiectasis, reticular veins, malleolar flare | Telangiectasis defined by dilated intradermal venules <1 mm in diameter Reticular veins defined by dilated, nonpalpable, subdermal veins ≤3 mm in diameter |
| C2 | Varicose veins | Dilated, palpable subcutaneous veins generally >3 mm in diameter |
| C3 | Edema without skin changes | |
| C4 | Skin changes ascribed to venous disease | |
| C4A | Pigmentation, venous eczema, or both | |
| C4B | Lipodermatosclerosis, atrophie blanche, or both | |
| C5 | Skin changes with healed ulceration | |
| C6 | Skin changes with active ulceration |
* Chronic Venous Disease grading system based on descriptive clinical, etiological, anatomical, and pathophysiological classification scheme.
 Central Venous Stenosis
Central Venous Stenosis
Superior vena cava (SVC) syndrome is a serious disorder resulting from impeded venous return from the upper body; it is caused by obstruction of the SVC. The symptoms include severe congestion and edema of the face, arms, and upper thorax and may progress to dyspnea, cognitive dysfunction, and headache. A clinical classification system that helps to classify the severity of symptoms (Table 43-2) has been used by several clinicians. The SVC syndrome is usually caused by obstruction, extrinsic or intrinsic, of the SVC, although bilateral obstruction of both brachiocephalic venous segments can result in a similar syndrome. Compression caused by mediastinal malignancy or lymphadenopathy is the most common cause of SVC syndrome.9 The syndrome can also result from extension of central deep venous thrombosis (DVT) to the SVC, usually in the presence of bilateral subclavian vein stenosis. Other benign etiologies include (1) thrombosis caused by underlying stenosis from long-term indwelling central venous catheters or other transvenous instruments and (2) benign compressive or constrictive conditions of the mediastinum, such as adenopathy from earlier histoplasmosis, fibrosing mediastinitis, previous irradiation, tuberculosis, and histiocytosis.10–14
TABLE 43-2 Clinical Scoring System for Central Venous Stenosis
| Signs and Symptoms | Grade |
|---|---|
| Neurologic symptoms | |
| Stupor, coma, blackout | 4 |
| Blurry vision, headache, dizziness, amnesia | 3 |
| Changes in mentation | 2 |
| Uneasiness | 1 |
| Laryngotracheal or thoracic symptoms | |
| Orthopnea, laryngeal edema | 3 |
| Stridor, hoarseness, dysphagia, shortness of breath | 2 |
| Cough, pleural effusion | 1 |
| Nasal and facial signs or symptoms | |
| Lip edema, nasal stiffness, epistaxis, rhinorrhea | 2 |
| Facial swelling | 1 |
| Venous dilation | |
| Neck vein or arm vein distension, upper extremity swelling or upper body plethora | 1 |
Modified from Kish K, Sonomura T, Mitsuzane K, et al. Self expandable metallic stent therapy for superior vena cava syndrome: clinical observations. Radiology. 1993;189:531-535.
Diagnosis of Central Venous Stenosis
The clinical presentation of SVC syndrome is relatively consistent and can be verified with multiple diagnostic modalities. Computed tomography (CT) is helpful for the workup of SVC syndrome and often gives enough information to proceed directly to an endovascular procedure.15 An upper extremity venogram will reveal multiple collaterals if the obstruction is of long standing. With acute SVC obstruction, however, there are often surprisingly few collaterals. In most cases, the venogram will demonstrate the level of involvement, but it can still overestimate the length of involvement of the innominate veins and even the SVC because of the high resistance to flow. Magnetic resonance imaging (MRI) is helpful for the evaluation of SVC syndrome, and procedure planning can be based solely on MRI findings.16 MRI will give good information regarding the extent of occlusion and collateral flow. Ultrasound, on the other hand, is not accurate in locating the central obstruction. The Doppler signal will raise a suspicion of obstruction because of the flattened character of the waveform, but the level of obstruction is difficult or impossible to estimate with ultrasound, especially in the central portion of the SVC. Ultrasound with Doppler can, however, be a useful tool for follow-up after endovascular repair. Obstructive changes in the waveform will raise suspicion of recurrent narrowing or occlusion of the recanalized vessel.
Technique
Endovascular therapy has emerged as first-line treatment for central vein stenosis.17 It is important to have a plan of approach before attempting SVC recanalization or stent placement. Taking into account the patient’s clinical presentation and preprocedural noninvasive imaging is essential to a successful outcome. Many operators have used femoral vein access, but others have used jugular, subclavian,18 and arm vein access or even transhepatic venous approaches for stent delivery.19 One may use any combination of these approaches, but it is always paramount to have a guidewire “through and through,” especially when stenoses are severe and difficult to pass.20,21 It certainly adds to the safety of the procedure to have a guidewire passing the right atrium into the inferior vena cava (IVC) during stent delivery and dilation. This is particularly important in preventing stent migration. This guidewire position will prevent the stent from migrating into the right side of the heart or pulmonary artery. The stent is thus more likely to stay on the wire and can be more safely manipulated, removed, or moved to a different location. Accessing the SVC from the right internal jugular vein or upper extremity veins has several benefits. Manipulation is easier because of the limited space in the internal jugular vein compared with the right atrium, which one has to work through in coming from a femoral vein access. The distance from the access site to the obstruction is also shorter, which can make it easier to cross chronic total occlusions. An upper extremity access, as via the brachial or basilic vein, is also a viable option. The entire venous intervention can be performed from this access, including thrombolysis, angioplasty, and stent placement in most cases.22 This is also comfortable and well tolerated by most patients. Hemostasis is not usually problematic, placing a pressure dressing for 20 minutes or holding pressure for 5 to 10 minutes is usually successful. If double-barrel stenting is required to treat the SVC, bilateral upper extremity access is ideal, with the stents each traversing the brachiocephalic veins. Several operators advocate using the common femoral vein for access, but it may be difficult to access an occluded or severely narrowed SVC from the femoral vein because of its anatomical relationship with the right atrium.23,24 Some operators have therefore crossed the obstruction from the brachial veins, creating a through-and-through access from the femoral vein by snaring the wire and pulling it through the femoral vein.25 In addition, double-barrel stents into each of the brachiocephalic veins can be easily placed from a bilateral common femoral vein access.
There is some experience in performing thrombolytic therapy prior to SVC stent placement as well as in conjunction with it. Isolated pharmacomechanical thrombolysis plus primary stenting is a combined procedure that opens an acutely thrombosed superior vena cava rapidly to alleviate symptoms in seriously ill patients with SVC syndrome. O’Sullivan26 reported a case series where all patients received isolated pharmacomechanical thrombolysis with tissue plasminogen activator delivered in a Trellis Peripheral Infusion System (Bacchus Vascular, Santa Clara, CA) that removed obstructive clots in minutes versus the 24 to 48 hours required for traditional catheter-directed thrombolysis. In each patient, stents were deployed immediately following pharmacomechanical thrombolysis in a combined procedure lasting less than 1 hour. In the appropriate clinical setting, thrombolysis for SVC syndrome prior to endovascular therapy may have great utility. By resolving the acute thrombus, one need only stent the underlying stenosis, which most of the time is shorter than the occluded segment that was occupied by thrombus. Therefore a shorter segment of vessel has to be stented, thus reducing the number of stents utilized and ultimately also reducing the potential thrombogenic stent surface. Gray, et al.27 reviewed the outcomes from thrombolysis of SVC thrombus in 16 patients; they had no major complications and complete success in 56% without stents. Thrombolysis was effective in 73%. Stents were not used in any of the patients, which may explain many of the poor clinical outcomes. Many patients with SVC syndrome have central venous catheters in place, on which they are dependent. Numerous reports describe pulling the catheters up from the SVC during the procedure and then placing the catheter down through the stent immediately after the procedure.28
Stent Selection
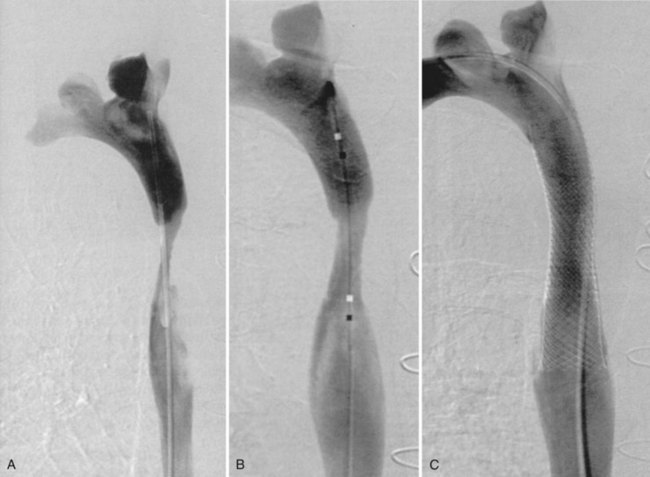
Early reports of SVC stenting described the use of Gianturco (Cook, Incorporated, Bloomington, IN) stents. This was the first self-expanding stent in wide use and had diameters that were acceptable. There were few complications reported, but large sheath introducers are required for this stent, and it is used only in cases where inflow vessels must be covered because of the large spaces between the stent’s interstices. Next, the Palmaz stent (Cordis Corporation, Miami, FL) was released for use. This stent is balloon-expandable and is now rarely used in the SVC. It has a higher radial force than self-expanding stents and, on occasion, when extra radial force is needed, can be used either primarily or secondarily within a deployed self-expanding stent that cannot sustain the radial force of SVC recoil. In today’s era of endovascular therapy, self-expanding stent delivery systems are used mainly for SVC stenting (Fig. 43-2).29,30 The newer self-expanding stent delivery systems are easily deployed and come on a small 6- to 7-Fr delivery system. The WALLSTENT (Boston Scientific Corporation, Natick, MA) was one of the earlier versions of a self-expanding stent and is still widely used for SVC intervention. Its main shortcoming is that it foreshortens significantly on delivery, which makes precise placement difficult. Recent generations of self-expanding stents are made of nitinol and do not have this problem. A few examples of self-expanding nitinol stents include the Smart Stent (Cordis Corporation, Miami, FL), Luminex (Angiomed/Bard, Karlsruhe, Germany), and Zilver (Cook Incorporated, Bloomington, IN). For the SVC, a stent 12 to 16 mm in diameter is usually adequate.
Complications
In a group of 59 patients with malignant disease, Lanciego reported 6 reocclusions, all of which were treated successfully with restenting in combination with thrombolysis.31 One of the patients in this series had stent migration to the right atrium, which was successfully treated.
Hemopericardium has been reported by several operators in the literature. This complication most commonly occurs during the procedure itself or immediately after stent placement,32 but delayed bleeding into the pericardium has also been described.33 The pericardial reflection can extend very high in the mediastinum, and the position is unpredictable.34 In this regard, it is advisable to place stents high in the SVC without compromising clinical results. If there is a high index of suspicion for hemopericardium, an echocardiogram or right heart catherization should be done immediately.
 Deep Venous Thrombosis of the Upper Extremity
Deep Venous Thrombosis of the Upper Extremity
Upper extremity deep vein thrombosis (UEDVT) is an increasingly important clinical condition with potential consequenes of significant morbidity and mortality. Subclinical pulmonary embolism may be present in 33% of patients with a UEDVT, but clinical pulmonary embolysis is detected in only 4% of such patients undergoing ventilation/perfusion scanning.35 The venous pathway of the upper limb is less likely to develop a DVT compared with the lower limb because of the relatively high rate of blood flow, gravitational effects, and lack of stasis.
Traditionally named after Paget and von Shroetter, UEDVT was typically regarded as a rare and benign condition. As available case reports and literature grew in the 1990s, UEDVT was increasingly seen as a more common and less benign disease potentially leading to serious complications, such as pulmonary embolism (PE), postthrombotic syndrome, and mortality.36–38 This change of view regarding UEDVT can certainly be explained by a higher degree of clinical awareness and the increased effectiveness and availability of noninvasive diagnostic techniques.39–41 Primary UEDVT is a rare disorder (2 per 100,000 persons per year)42 that refers either to effort thrombosis (Paget-Schroetter syndrome) or idiopathic UEDVT. Patients with Paget-Schroetter syndrome develop spontaneous UEDVT, usually in the dominant arm, after strenuous activity such as rowing, wrestling, weight lifting, or baseball pitching, but they are otherwise young and healthy.43 The heavy exertion causes microtrauma to the vessels’ intima and leads to activation of the coagulation cascade. Significant thrombosis may occur with repeated insults to the vein wall, especially if mechanical compression of the vessel is also present.44 Thoracic outlet syndrome involves compression of the neurovascular bundle (brachial plexus, subclavian artery, subclavian vein) as it exits the thoracic inlet.45 Although this disorder may initially cause intermittent positional extrinsic vein compression, repeated trauma to the vessel can result in the formation of dense perivascular fibrous scar tissue that will persistently compress the vein.45 Compression of the subclavian vein typically develops in young athletes with hypertrophied muscles who do heavy lifting or completely abduct their arms. Cervical ribs, long transverse processes of the cervical spine, musculofacial bands, and clavicular or first rib anomalies are sometimes found in these patients. Therefore plain films of the cervical spine and chest should be obtained in all patients undergoing evaluation for thoracic outlet syndrome.45 Presenting signs and symptoms of UEDVT can be found in Table 43-3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree