INDICATIONS/CONTRAINDICATIONS
Indications
Thoracoscopic or video-assisted thoracic surgery (VATS) left lower lobectomy has similar indications/contraindications, preoperative planning, positioning, and technique to right-sided lower lobectomy. Left lower lobectomies account for up to 15% of all lobectomies according to a recent series. The most common indication for VATS left lower lobectomy is primary nonsmall cell lung carcinoma (NSCLC). Approximately 170,000 deaths each year are attributable to lung cancer, surpassing the next four most common cancers combined.
For early staged cancers, complete surgical resection remains the cornerstone of curative therapy for NSCLC. An anatomic resection; lobectomy, segmentectomy, or pneumonectomy; is the standard surgical approach for stage I and II NSCLC. Nonanatomic wedge resection is often reserved for those patients with suitable tumor location and significant cardiopulmonary comorbidities that preclude a formal anatomic resection. Other indications for thoracoscopic left lower lobectomy include: Pulmonary metastases not amenable to sublobar resection, benign lung tumors not amenable to wedge resection due to anatomic considerations, congenital lesions such as arteriovenous malformations and pulmonary sequestrations, and infectious or inflammatory pathologies.
Contraindications
Contraindications to VATS are growing increasingly rare for experienced thoracoscopic surgeons. However, according to a recent publication analyzing data submitted to the Society of Thoracic Surgeons General Thoracic Database, only approximately one-third of all lobectomies are being performed thoracoscopically. The following are relative contraindications to proceeding with thoracoscopic left lower lobectomy: Necessity of bronchial sleeve resection to achieve negative margins; locally advanced tumors with need for concurrent chest wall, diaphragm, or major vascular resection with reconstruction; and preoperative chemotherapy or radiation therapy. Absolute contraindications for VATS lobectomy include very large tumors requiring a sizable thoracotomy for specimen extraction, the inability of the patient to tolerate single lung ventilation, and the surgeon being uncomfortable with performing the procedure. We feel that this last contraindication has resulted in the lack of widespread adoption of this technique; thoracoscopic lobectomy is more technically demanding than that done via thoracotomy.
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
There are two components in assessing the patient that presents with pathology requiring pulmonary resection, especially those requiring the removal of at least a lobe or greater of pulmonary parenchyma. The first part centers on the appropriateness of the intended surgical intervention from an oncologic standpoint. Patients with biopsy-proven lung carcinoma or those with suspicious pulmonary lesions believed to be malignant should undergo thorough clinical staging. In addition to conventional CT imaging, positron emission tomography (PET) should be employed to evaluate for evidence of regional or distant metastatic disease. Concerning extrathoracic abnormalities found on PET imaging should be considered for tissue sampling to disprove stage IV disease. Surgical or endoscopic staging of the mediastinum to rule out stage III disease should be contemplated for those patients with PET abnormalities in the hilar or mediastinal lymph nodes or those with large, central tumors. When compared to NSCLCs in other lobes, left lower lobe carcinomas have a higher prevalence of lymph node drainage to the contralateral mediastinum. As a result, patients with left lower lobe lung cancer who demonstrate right paratracheal lymphadenopathy by CT or PET criteria should undergo tissue sampling to exclude regionally advanced disease. National Comprehensive Cancer Network guidelines have been established allowing for appropriate preoperative staging and management of patients with NSCLC.
The second component in the evaluation of patients presenting with pulmonary pathology requiring lobectomy involves preoperative cardiopulmonary risk assessment. Despite the tremendous advances made in surgical technique, anesthetic management, and postoperative care, the overall risk of perioperative morbidity and mortality after lobectomy remains relatively high. The ability to predict those patients that are at significant risk allows physicians to appropriately select patients for lobectomy. There have been many useful studies identifying factors associated with major morbidity and mortality in patients undergoing pulmonary lobectomy.
The scope of this chapter precludes an expansive review of this data. We suggest performing a careful history and physical examination accompanied by spirometry and measurement of diffusion capacity (DLCO) along with arterial blood gas sampling. Predicted postoperative forced expiratory volume in 1 second (FEV1) and DLCO expressed as a percentage of normal are calculated by utilizing the number of pulmonary segments to be removed. For postoperative predicted FEV1 or DLCO less than 40% predicted, quantitative pulmonary perfusion imaging is performed for a more accurate calculation. For those patients with concerning results, exercise testing can be completed to determine oxygen consumption during maximum exercise. Other adjunct tests that may be useful include cardiac stress testing and echocardiography. Nevertheless, absolute numbers should not be the sole factor in determining the best treatment for a patient; the importance of sound clinical judgment cannot be overstated.
 SURGERY
SURGERY
The VATS lobectomy operation consists of individual hilar ligation via three to four incisions without rib spreading. Thoracoscopic lobectomy should duplicate the oncologic principles as those attained via traditional thoracotomy. That is, resection of tumor with negative margins while performing individual vascular and bronchial division with complete hilar lymph node dissection. In addition, a mediastinal lymph node dissection, or sampling, is performed. Certain aspects in VATS lobectomies, such as avoiding rib spreading, are stressed with the goal to improve the patient’s postoperative recovery. One variant of thoracoscopic lobectomy is the “video-assisted simultaneously stapled lobectomy.” This iteration does not employ individual bronchovascular ligation and is, therefore, basically a different operation and will not be discussed in this chapter.
There are some unique aspects to a left lower lobectomy compared to a right-sided lower lobectomy. These include adjusting the camera port to the posterior axillary line to avoid the heart, sampling level 5 and 6 nodes while avoiding recurrent laryngeal and phrenic nerve injury, and understanding the relationship of the lower lobe vein to the upper lobe vein while avoiding division of a common venous trunk. Standard thoracotomy instruments are generally the ones used for VATS lobectomies: Harken’s clamp, Pearson’s scissors, suction, sponge sticks or dental pledgets, Roberts or Fraser-Kelly clamps, straight or curved Foerster ring clamps. We also utilize a suction/irrigation device and endoscopic Kittner dissectors and stapling devices.
Positioning
Positioning for VATS left lower lobectomy, as in all minimally invasive surgery, is extremely important. Once anesthetized and intubated with either a single- or double-lumen endotracheal tube, careful bronchoscopic examination of the airway is performed to evacuate all secretions and to ensure that no endobronchial tumor is present. Once that has been completed, we prefer using a bronchial blocker, passed through the end of the single-lumen tube, to obtain lung isolation. An alternative method for obtaining lung isolation is through the use of a double-lumen endotracheal tube. The patient is then placed in the right lateral decubitus position and then taped in place after table flexion. The patient’s anterior-superior iliac crest is positioned at the level of the break of the bed and the table is optimally flexed to open up the intercostal spaces. The patient’s hips are rotated slightly posteriorly to minimize the camera from hitting the hips when looking more anteriorly. An axillary roll is placed under the chest to protect the axilla. The upper arm and shoulder are abducted as far above the head as possible while being sure to prevent brachial plexus stretch (approximately 100 degrees). Prior to prepping, double-lumen tube position or location of the bronchial blocker is reconfirmed with bronchoscopy. Two video monitors are placed at the right and left sides of the head of the bed.
Technique
After prepping and draping, we begin by placing the first of three ports (Fig. 18.1). The camera port, placed in the eighth intercostal space, is aligned with the posterior axillary line. Again, no rib spreading is done. We generally use a metal thoracoscopic port for our 30-degree 10-mm thoracoscope. We almost exclusively use a 30-degree thoracoscope. It provides optimal views not afforded by a 0-degree scope, particularly during the difficult dissection around the superior hilum.
It is important to be aware of a possible elevated left hemidiaphragm and to use the lateral chest radiograph to help make adjustments of this port placement either more anterior, or more posterior, or even one rib space up on occasion. Injuries to the diaphragm can occur in an obese patient or one with an elevated hemidiaphragm if the correct approach is not taken.
Our second port is usually placed in the fourth or fifth intercostal space, either inferior or posterior to the scapular tip. This port usually serves as the lung retraction port and for placement of the suction/irrigation device. This is done under direct vision. Some muscles are divided with the cautery and we dissect directly down to the intercostal space. Care is taken not to injure the corresponding intercostal neurovascular bundle.
We then use the camera and lung retraction via the posterior port to choose the location of the final port site. This anterior port should be placed right over the hilum since this will be used as the access port. Dissection of both the hilum and the fissure will be performed through this port. This incision is initially 1 to 2 cm. It is not extended to 5 cm in length until we have decided to proceed with the VATS lobectomy. The port is usually created anterior to the latissimus dorsi in the fifth intercostal space for left lower lobectomy. A 22-gauge spinal needle may be useful to properly localize the optimal interspace in obese patients. This is an area relatively devoid of major musculature as it is located posterior to the pectoralis major muscle and anterior to the latissimus dorsi. There are usually intercalations of serratus anterior in this area. Hemostasis is very important when creating the ports as bleeding from the port sites onto the camera and onto the surgical field during the procedure is a nuisance and can significantly prolong the operation.
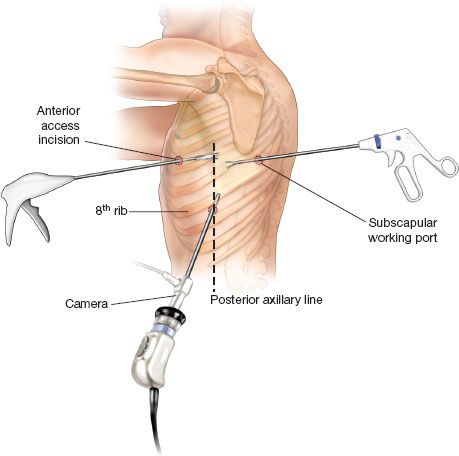
Figure 18.1 Port Placement. Camera port is placed in the eighth intercostal space in the posterior axillary line. The subscapular working port is placed in the interspace just posterior to the tip of the scapula. The anterior access port is placed at the fourth or fifth intercostal space in the anterior axillary line. The exact location is based on visualization of the anterior hilar structures.
Prior to beginning the dissection, the pleural cavity is thoroughly inspected. The pleural surfaces are explored for metastases and any adhesions are then divided. As in open surgery, total lysis of adhesions is completed to restore the normal anatomy of the left lung. This helps avoid judgment errors regarding anatomic anomalies, allows the remaining lung to occupy the pleural space after resection, and generally makes the rest of the case proceed more expeditiously. Adhesions are divided either sharply, with cautery, or with an ultrasonic cutting and coagulation device. An endoscopic Kittner dissector may be passed through the camera port to provide countertraction against the lung when dividing adhesions between the lower lobe and diaphragm. Again, full mobilization of the lung is vital to effectively complete the lower lobectomy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


