Chapter 28 Vasculogenic Erectile Dysfunction
Introduction
The first historical descriptions of erectile dysfunction (ED) date back to Egyptian papyrus nearly 4000 years ago. Egyptian scholars described two types of ED: a “natural” form in which the man was incapable of performing the sex act, and a “supernatural” form rooted in evil charms and spells.1 Ancient thinkers such as Hippocrates and Aristotle also theorized on the etiology of ED. However, the first accurate depiction of penile anatomy and rudimentary analysis of erection was not published until 1585, when Ambroise Paré described it in his Ten Books on Surgery and the Book of Reproduction.2 In these texts, Paré portrayed the penis as a tube with concentric coats of nerves, veins, arteries, two “ligaments” composed of the corpora cavernosa, and the urinary tract.
Definition and Classifications
In 1992, the National Institutes of Health convened a Consensus Development Conference on Impotence. The group renamed impotence as male erectile dysfunction and defined it as “the inability to achieve or maintain an erection sufficient for satisfactory sexual performance.”3 Furthermore, they noted that erectile dysfunction represents the most appropriate term, given that sexual desire, orgasm, and ejaculation may be intact despite inability to achieve or maintain erection.
Multiple schema have been proposed to classify the different types of ED. Broadly, ED can be described in terms of organic and psychogenic dysfunction (Box 28-1). The main thrust of this chapter will center on vasculogenic ED, which comprises impaired endothelial function, arterial occlusive disease, veno-occlusive dysfunction, and structural changes to the corpora cavernosa.
Prevalence and Incidence
Erectile dysfunction is quite common, affecting approximately 30 million men in the United States.4 Several population-based studies have been performed to address male sexual function and specifically the prevalence and incidence of ED in the American male population. The 1992 National Health Social and Life Survey (NHSLS) was a national survey of 1410 American men between the ages of 18 and 59. In the study group, the prevalence of ED in men aged 18 to 29 years was 7%, aged 30 to 39 was 9%, aged 40 to 49 was 11%, and aged 50 to 59 was 18%.5 The Massachusetts Male Aging Study (MMAS), a longitudinal population-based study, evaluated 1709 men between the ages of 40 and 70 who returned questionnaires about a broad range of physiological measures, demographic information, and self-reported sexual function. Participants were surveyed between the years 1987 and 1989 and then reevaluated between 1995 and 1997. In this series, the age-adjusted prevalence of significant ED was 39% in men with coronary artery disease (CAD), 25% in men with diabetes mellitus, and 15% in men with hypertension. Incidence of ED on reevaluation was 25.9 cases per 1000 men per year (95% confidence interval [CI], 22.5-29.9).6 Using these data, it was estimated that for Caucasian men, 617,715 new cases of ED would present in the 40 to 69 age group each year.7 Data from European and Brazilian researchers suggest a similar incidence of ED in their respective countries.8,9
Functional Anatomy
Corporal Bodies, Sinusoids, and Glans
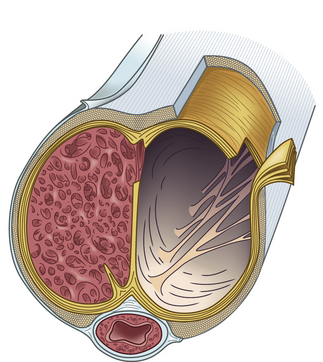
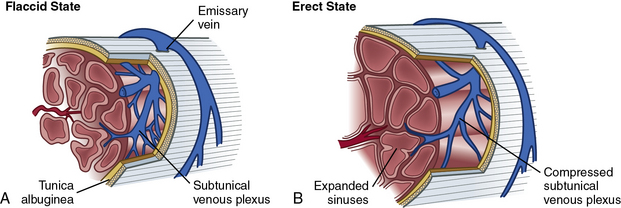
Within the corpora, interconnected sinusoids are enveloped by trabeculae of smooth muscle, collagen, and elastin (Fig. 28-1). The sinusoidal smooth muscle is in intimate association with the cavernous nerves and helicine arteries within the penis. The sinusoids are tonically constricted during the flaccid state. Arterial blood flow diffuses through larger central sinusoids to smaller peripheral sinusoids. In the flaccid state, this slow diffusion of arterial blood results in blood gas values similar to venous blood. During sexual stimulation, release of neurotransmitters causes the smooth muscle around the sinusoids to relax. This results in rapid influx of arterial blood, subsequent entrapment of blood within these expanding sinusoids, and occlusion of veins traversing the tunica albuginea. Subsequent tumescence results in pressure increases of several hundred mmHg and blood gas values approaching arterial levels.10
Tunica Albuginea
The tunica albuginea is composed primarily of tough type I collagen with a minority component of more flexible type III collagen and elastin. It is arranged in a bilayer, with inner circular layers and outer longitudinal layers (see Fig. 28-1). Intervening struts traverse the body of the corpora cavernosa and provide further support.11 The longitudinal layers of the tunica are present from the glans to the proximal crura, where each corporal body inserts into its ischial ramus to form a foundation for support of the erect penis. Emissary veins (Fig. 28-2) pierce the tunica albuginea. During engorgement, these veins become compressed and allow entrapment of blood within the penis.
Arterial System
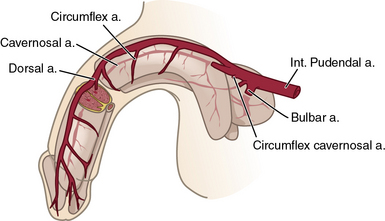
The internal pudendal artery, a branch of the internal iliac artery (IIA), is the principal source of blood flow to the penis. Up to 70% of men may have accessory pudendal branches that originate from the external iliac, obturator, or vesical arteries.12 The internal pudendal artery gives rise to the penile artery, which in turn branches in to the dorsal, bulbourethral, and cavernous arteries (Fig. 28-3). The cavernous artery supplies the corpus cavernosum via helicine arteries, which lie in close approximation to the sinusoidal tissue. During erection, these vessels dilate, resulting in engorgement.
Venous System
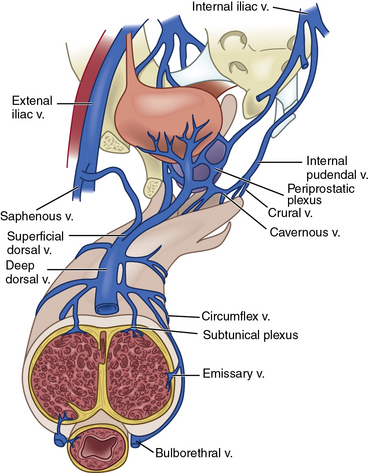
Venous drainage originates from the three corporal bodies. Venules interdigitate through the cavernosal sinusoids and coalesce below the tunica albuginea into a subtunical plexus. The plexi then form emissary veins that penetrate the tunica albuginea. From there, numerous subcutaneous veins course along the shaft of the penis to form the superficial dorsal vein and a deep dorsal venous system, which in turn drain into the saphenous vein and retropubic venous plexus, respectively13 (Fig. 28-4; also see Fig. 28-2).
Nervous System
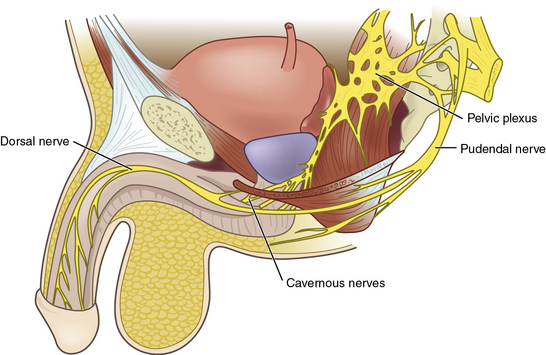
Penile innervation occurs via both autonomic (parasympathetic and sympathetic) and somatic (motor and sensory) pathways. Erection and detumescence are largely regulated via the autonomic system. Sympathetic and parasympathetic nerves coalesce to form the cavernous nerve, which penetrates the corpora cavernosa to exert its effect on erection (Fig. 28-5). Sensation and contraction of penile musculature occurs via the somatic nerves.
Autonomic pathways
Between the T11 and L2 spinal segments, the sympathetic trunk begins. These fibers then form the sympathetic chain ganglia, which continue caudally to the inferior mesenteric and superior hypogastric plexi. Further sympathetic fibers exit to form the hypogastric nerves, and ultimately the sympathetic portions of the pelvic plexus.14
Between the S2-S4 spinal cord segments, the parasympathetic pathway originates. These fibers also continue caudally to the pelvic plexus (see Fig. 28-5), where they join the aforementioned sympathetic nerves. Together, these nerves then join to form a network of nervous tissue that passes along the lateral and posterior aspect of the prostate to create the cavernous nerves.15 Stimulation of the sympathetic trunk via the cavernous nerves results in detumescence. Excitation of the parasympathetic aspects of the pelvic plexus and cavernous nerves is responsible for erection. To avoid iatrogenic ED, clear understanding of the location of these nerves is critical during pelvic surgery such as radical prostatectomy or abdominal perineal resection.
Somatic pathways
Sensory receptors in the penile skin and glans are unique in the human body.16 They are composed of free nerve endings comprising unmyelinated C fibers and thin myelinated A-delta fibers. These coalesce into the dorsal nerve of the penis, which ultimately forms the pudendal nerve. The pudendal nerve then enters the S2-S4 nerve roots at the spinal cord. Via spinothalamic and spinoreticular pathways, sensations such as touch, pain, and temperature are perceived.17 Interestingly, research by Burnett et al.18 suggests that the dorsal nerve of the penis carries both autonomic and somatic signals, and therefore contributes to penile sensation, erection, and ejaculation.
Pathophysiology of Erectile Dysfunction
Vasculogenic Erectile Dysfunction
As noted in Box 28-1, ED often represents a multifactorial disease state. Although the focus of this chapter is on the vasculogenic determinants of ED, it is worth noting that within an individual patient, neurological, hormonal, or psychological etiologies of ED may be of contributory or even primary importance. With that said, it is clear the vascular system is responsible for providing blood flow to the erectile tissues of the penis, so any dysfunction within the vascular system may affect erectile function.
Arteriogenic erectile dysfunction
Arteriogenic ED can be due to atherosclerotic or traumatic arterial occlusive disease. Michal and Ruzbarsky19 noted impaired penile perfusion is an indicator of generalized atherosclerotic disease, and that the age of onset of ED and CAD is often similar. In fact, ED has been shown to be a bellwether for development of CAD in asymptomatic men,20 and both diseases share the same risk factors—specifically smoking, diabetes, hypercholesterolemia, and hypertension.21
In arteriogenic ED, the corpora cavernosa demonstrate lower oxygen tension,22 which may result in a decreased volume of sinusoidal smooth muscle and subsequent venous leak.23 In an experimental animal model, rabbits with iatrogenic iliac atherosclerotic disease demonstrated alterations in their downstream penile arteries and a reduction in cavernosal smooth muscle content.24 These alterations were associated with decreased nitric oxide synthase (NOS)- and NO-mediated relaxation of corpora cavernosal tissue.25 Erectile dysfunction due to traumatic stenosis of cavernous or pudendal arteries has been noted in young men with pelvic trauma26 and in long-distance cyclists.27
Venogenic erectile dysfunction
Not only can diabetes, hypertension, hypercholesterolemia, and penile injury result in penile arterial disease, these disorders can also result in loss of elastic fibers within the cavernosal venules and sinusoids. This loss of compliance results in diminished venous trapping and subsequent veno-occlusive dysfunction.28 In fact, diminished venous occlusion may represent the most common form of vasculogenic ED.29 Loss of smooth muscle relaxation due to heightened adrenergic tone or decreased NO release may exacerbate already poor compliance in these fibrotic sinusoids.30 Finally, fibrosis leading to increased collagen deposition between cell membranes may abolish critical signaling and intercellular transmission via disrupted gap junctions.31
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree