Chapter 9 Vasculogenic Erectile Dysfunction
Impotence, or male erectile dysfunction (ED), is the persistent or repeated inability to attain and maintain an erection sufficient for satisfactory performance in the absence of an ejaculatory disorder.1 ED, now better understood and more effectively treated than in the past, is important in the diagnosis and treatment of vascular disease. Progress in this field includes comprehension of normal erectile physiology, understanding of stimuli promoting cavernous sinus smooth muscle relaxation,2 and development of effective treatments for ED.3 Vascular surgeons will be mainly concerned with prevention of ED and other sexual dysfunction in relation to aortoiliac interventions. They should be able to delineate the varying etiologies of ED as well as vasculogenic ED, and recognize that aortoiliac reconstruction itself can cause ED by failing to perfuse the internal iliac arteries or by damaging autonomic genital nerves.4 Techniques of concern for vascular surgeons are those that minimize or completely avoid damage to the pelvic nerves and restore or maintain internal iliac artery perfusion. Familiarity with these techniques can prevent sexual dysfunction and, in some instances, restore potency after aortoiliac interventions.5,6
This chapter describes surgical approaches for the prevention of postoperative ED during aortoiliac interventions. Vasculogenic ED owing to small vessel disease, cavernous smooth muscle dysfunction, and primary ED are also common disorders. The vascular specialist should also be aware of evolving approaches to medical and surgical treatment of male sexual function7 and recognize female sexual dysfunction (FSD) caused by vascular disease, following aortoiliac surgery8 and after radical hysterectomy.9 Nerve-sparing and revised operative techniques are available to prevent or minimize these effects.10,11 Indications for and results of microvascular procedures are summarized along with medical treatment for ED, which is a rapidly expanding area of interest. More than 16,500 citations about ED appeared in PubMed since the 1940s, and more than 180 reports were published within the first quarter of 2011.
Physiology of Erection
Penile erection requires adequate arterial inflow and closure of cavernosal outflow, mediated by a complex interplay between neural and local factors.12,13 Erection results primarily through relaxation of the smooth muscle of the corporal bodies. Endothelial-mediated relaxation responses stimulated by neural mechanisms function to open the penile cavernous spaces.14 Nitric oxide (NO) as the chemical mediator15 and increasing arterial blood flow with oxygenation of the cavernous smooth muscle16 promote the erectile process. As intracavernosal flow increases, a greater amount of oxygen stimulates additional NO synthesis by cavernosal nerves and endothelium. Cavernosal oxygenation promotes penile erection, whereas hypoxemia is inhibitory. Testosterone, in addition to its central effects, stimulates NO synthase activity in corporal tissues,17 thus enhancing sensitivity to cavernosal nerve stimulation. NO, in turn, activates conversion of guanosine triphosphate to cyclic guanosine monophosphate (GMP). The latter provides the message leading to relaxation of the smooth muscle within the corpora cavernosa.18 Agents inhibiting hydrolysis of cyclic GMP increase messenger cyclic GMP, thus facilitating smooth muscle relaxation, which promotes penile erection.19 Cyclic nucleotide phosphodiesterase (PDE) isoenzymes increase hydrolysis of cyclic GMP; among these, PDE-5 and PDE-6, which are specific for the substrate in human cavernosal tissue.20 Inhibition of PDE-5 is a key means of treatment for ED. As corporal arterial pressure increases, draining emissary veins are compressed against the tunica albuginea causing venous outflow occlusion. During full erection, cavernosal artery flow virtually ceases. During flaccidity, a constant venous leak balances baseline penile inflow and outflow. With insufficient arterial inflow, the corpora fail pressurize adequately, and secondary venous leakage occurs. Intracavernous pressure increases from 10 to 15 mm Hg to levels ranging from 80 to 90 mm Hg in the erect state. Intracavernous pressures higher than systemic pressure, generated by perineal muscle contraction,13 contribute to penile rigidity.
Investigation of the Complaint of Erectile Dysfunction
Box 9-1 summarizes general factors contributing to erectile dysfunction; the complaint of ED occuring preoperatively and, of more concern, postoperatively. In screening 1023 men with the chief complaint of impotence, 461 demonstrated an arterial inflow problem, based on noninvasive criteria using the penile brachial index and pulse volume recordings.21,22 However, many men exhibited other contributing factors, including diabetes, neuropathy (approximately 20%), antihypertensive medication, and cavernous dysfunction, including Peyronie disease. Older men with multiple factors contributing to ED are generally not candidates for vascular surgical intervention for this complaint alone. Approximately 6% to 7% of the men investigated for ED ultimately became candidates for vascular interventions. In this experience, only 15.6% of men with decreased arterial perfusion were found to have large vessel disease. Imposing a selective screening sequence for surgical case selection yields a sharp funnel effect that minimizes candidacy for vascular intervention for ED.22 Although proportionate vascular involvement is not high, for young men with small vessel disease or trauma or macrovascular disease, vascular intervention is a logical first step for those failing medical therapy and for those who do not desire prosthetic implantation.23
Box 9-2 offers an updated classification of vasculogenic ED. Some type of small vessel, cavernosal, or arteriolar cause is present in 43.3% of men exhibiting abnormal penile perfusion. An additional 41.1% of men with the primary complaint of impotence exhibit a combination of large and small vessel involvement, as ascertained by noninvasive and physical criteria. Most men with the primary complaint of ED are more likely to have small vessel or cavernosal disorders than macrovascular disease. Importantly, the complaint of impotence is associated with occult aortoiliac occlusive or aneurysmal disease.
Box 9-2
Classification of Vasculogenic Erectile Dysfunction
• Large vessel aorta and branches to internal iliac artery
• Small vessel anterior division of internal iliac artery and penile arteries
• Combined atheroembolism from aortoiliac segment
• Fibrosis postpriapic, drug injection, idiopathic with aging
• Peyronie’s disease deformity; venous leakage
• Refractory states hormonal, diabetic, blood pressure medication
• Acquired various patterns; dorsal vein, crural, spongiotic
In men with Leriche syndrome,24,25 impotence as a sentinel complaint sometimes precedes the onset of claudication. Atheroembolism from ulcerated plaques or occult aneurysms can cause sudden onset of erectile dysfunction.21 Men younger than 55 years are often potent before reconstruction for aneurysm; an accurate history of their sexual activity must be obtained. Despite the best surgical techniques to preserve sexual function, ED and other sexual disabilities continue to occur after reconstructions for aneurysms and occlusive disease. Therefore, before intervention, the surgeon must make careful inquiries into the patient’s sexual function and, when necessary, assess preoperative penile artery perfusion. A detailed history and noninvasive testing are particularly important when postoperative sexual function is an expressed concern of the patient.
History and Physical Findings in Erectile Dysfunction
A history of gradual erectile failure, in the absence of traumatic life events occuring with intermittants claudication, suggests large vessel arteriogenic ED. In these men, both the intensity and the duration of atherosclerotic risk factors, mainly cigarette smoking, hypertension, diabetes, and hypercholesterolemia, contribute to vascular disease. This pattern signals patients who have involvement of the aorta or the iliac system. As mentioned, abrupt onset of erectile failure can be due to emboli from abdominal aneurysms or ulcerated aortoiliac disease.21 Perineal injury and pelvic fractures can cause arteriogenic impotence. The immediate onset of erectile failure after urologic, vascular, or rectal operations suggests neurovascular damage. Although either neural or vascular interruption can cause ED, periaortic, sympathetic, or hypogastric neural interruption can cause ejaculatory disorders. Alcohol and drug abuse contribute to progressive erectile failure, including drugs used to treat hypertension, some antiinflammatories, and sedatives. Hormonal disorders, such as hypogonadism, rarely cause ED. Our group detected two prolactinomas during the screening of approximately 1400 men; these men exhibited dramatic responses to medical therapy.
Decreased femoral pulses or bruits or palpation of an abdominal aortic aneurysm in those whose waists measure less than 38 to 40 inches confirm aortoiliac involvement. Sensory testing of the extremities, perineum, or glans may reveal neuropathies associated with diabetic impotence. However, these abnormalities are most reliably quantified by neurovascular testing, using pudendal evoked potentials and measurement of bulbocavernosus reflex times.26,27 Currently, neurologic screening is initially deferred; medical treatment with vasoactive agents is often effective, even with associated neuropathy. In cases of postoperative or posttraumatic dysfunction, neurologic testing documetnting a deficit guides decision making, particularly in recommending prompt prosthetic implantation as a treatment option.
At this point, the erectile mechanism can be tested in the clinic by intracavernous injection of Prostaglandin E1 (10 to 20 µg).28 Rigid erection sufficient for intercourse demonstrates adequate arterial inflow and venoocclusive mechanisms. Provided that aneurysmal disease has been ruled out by sonography, initial treatment is with oral, injectable, or intra-uretheral vasoactive agents, which may be effective in up to approximately 60% of cases depending on population demographics and underlying patterns of disease.
ED is a symptom, not a single disease. The factors summarized in Box 9-1 help to guide approaches to the diagnosis and treatment of sexual dysfunction and, in some respects, are relevant to both sexes. An important area has been the study of FSD, which appears in women with diabetes29,30 and cardiovascular disease31 and in postmenopausal women as disordered sexual arousal,32 failure to achieve orgasm, and dyspareunia with failure to lubricate. Feminine arousal and vaginal lubrication are difficult to measure, whereas penile erection can be readily seen and quantified. Progress in understanding the cause, physiology, and treatment of FSD continues.33 Recently, variable results of aortic surgery in women have been described.34,35 Treatment of FSD with PDE-5 inhibitors is not recommended; however topical alprostadil has been proved effective in a recent randomized trial.36 From the standpoint of aortic reconstruction, the potential benefits of nerve-sparing dissections with preserved internal iliac flow for both sexes should be recognized.
No universally accepted approach for diagnosis and management of ED exists; as mentioned, ED is a symptom rather than a single disease. Ideally, correction of the underlying condition should be attempted. In current practice, the patient’s goals tend to direct therapy. In the absence of aneurysmal disease, further treatment will depend on the initial response to medical therapy.37 When oral medication with PDE-5 inhibitors fails, more elaborate investigations can be considered.38 Should the intracavernous administration of vasoactive agents fail and vacuum constrictor devices prove ineffective, and if vascular intervention is an option, evaluation may progress to more elaborate invasive tests to delineate more subtle anatomic vascular abnormalities. Depending on circumstances, treatment might also progress directly to prosthetic implantation.
Neurovascular Testing
Neurovascular testing, initially used for all patients by our group22 to screen candidates for reconstructive procedures,23 was found useful in determining an initial dose for intracavernous injection of vasoactive agents. Patients with neurologic deficits were discovered to be exquisitely sensitive to injection of intracavernosal agents with a heightened risk of priapism. With availability of orally administered PDE-5 agents, such testing was less frequently needed. Comprehensive testing is useful for the investigation of postoperative onset and ED owing to perineal trauma, and occasionally to resolve legal issues.
The penile brachial index (PBI), the ratio between systolic pressure detected by a Doppler probe placed distal to a penile cuff and systemic or brachial arm pressure, is a convenient, easily accomplished perfusion measure.39 A plethysmographic cuff of 2.5 cm, used for an average-size penis, is inflated, then deflated. The reappearance of Doppler signals in the dorsal artery branch proximal to the corona signals reflow. Normally, this pressure approaches systemic pressure. A PBI greater than 0.75 suggests that no major obstacle exists between the aorta and the distal measurement point. Generally, PBIs less than 0.6 relate to major vascular obstructions in the aortoiliac system, while PBIs between 0.6 and 0.75 are considered abnormal.
These noninvasive tests have limited sensitivity and specificity. The combination of PBI and pulse volume recording predicts an abnormal arteriogram with a sensitivity of 85% and a specificity of 70%. In suspected cases of venogenic impotence (i.e., normal arterial noninvasive tests), 23% of men examined with normal noninvasive studies had associated arterial lesions demonstrated angiographically.40 Therefore, before microvascular interventions are performed for those failing medical therapy,18 both pudendal arteriography and dynamic infusion cavernosography are required for proper case selection.
PBI detects inadequate arterial inflow from large arteries, but does not detect vasculogenic impotence caused by venous leak, Peyronie disease, or cavernosal fibrosis. In these instances, color-flow duplex scanning after an intracavernous injection to produce erection or tumescence has been used mainly by urologists, sometimes in combination with visual erotic stimulation, to measure deep cavernosal and dorsal blood flow velocity at intervals after the injection of a vasodilator.41 Based on these studies, ED can be classified as arterial, venous, or mixed vascular. Studies of local blood flow dynamics yield little information about aortoiliac anatomy. Nocturnal penile tumescence and rigidity monitoring, using noninvasive strain gauge techniques, are performed in a sleep laboratory over several nights. Home monitoring devices are available. A normal rigid erection observed during sleep rules out organic impotence.42
Cavernosometry and Cavernosal Artery Occlusion Pressure
Invasive studies provide quantitative information about arterial inflow and venoocclusive mechanisms.43 A calibrated pump provides a flow of warm, heparinized saline via 20-gauge needles inserted into the corpora. During maximal erection, intracavernous pressure at some point equilibrates with arterial inflow pressure, and flow in the deep cavernosal artery stops. This value is called cavernosal artery occlusion pressure; it is measured with Doppler insonation at the point of full erection. Normal pressure is greater than 90 mm Hg. A pressure gradient from brachial levels greater than 30 mm Hg suggests arterial inflow occlusion. Dynamic infusion cavernosography measures the flow required to maintain erection. This value is normally 40 mL or less after intracavernous injection of a standard papaverine-phentolamine mixture. Nonionic dilute contrast is injected to visualize venous leaks. Spot filming in various obliquities identifies specific abnormal or leaking veins when cavernosography is positive. As mentioned previously, failure of erection can be caused by an excess of venous leakage over inflow. Venous leakage also can be a seondary effect of arterial insufficiency. Therefore selective pudendal arteriography should be done before venous ablation.
Aortoiliac Reconstruction Principles
Given the standard indications for large vessel aortoiliac reconstruction (i.e., aneurysm or occlusive disease), the procedure, whether endovascular44,45 or open,46,47 should be planned to provide perfusion of both internal iliac arteries whenever possible. Flushing of debris into the internal iliacs should be avoided, and endovascular repair should attempt to maintain internal iliac flow—at least to one internal iliac artery.17 The dissection in open cases must spare the neural fibers about the aorta and the iliac arteries (which are especially rich on the left side) and about the inferior mesenteric artery. In all these cases, a specific history of preoperative sexual activity must be sought. If an elderly person manifests no interest in sexual activity, complicated preoperative testing is unnecessary. However, when sexual interest exists, preoperative PBI and pulse volume recordings are helpful for later postoperative blood flow assessment.
Operative Techniques
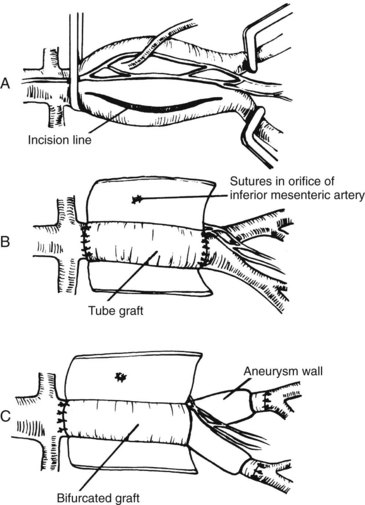
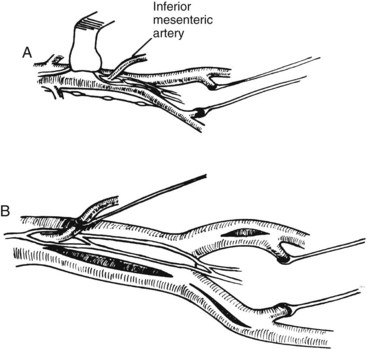
Operative techniques to spare autonomic nerves and to preserve or restore internal iliac blood flow have been published.46,47 The two basic principles described previously apply equally to endosvascular and open procedures. Open aortoiliac reconstruction can be accomplished by dissecting the aortoiliac segment from the right and sparing the nerves and inferior mesenteric artery. In cases of aortoiliac aneurysm, perfusion of the internal iliac is ensured by an inlay technique (Figure 9-1). Again, the aneurysmal sac is incised well to the right, avoiding interruption of a dominant left periaortic nerve plexus. The inferior mesenteric artery is sutured from within the aneurysmal sac. Figure 9-2 shows techniques of exposure for endartectomy or bypass for occlusive disease. In men with buttock claudication and impotence related to local disease in the arterial distribution of the internal iliac artery, an extraperitoneal approach with endarterectomy or bypass is not a difficult procedure, although endovascular interventions have been used. The open approach uses a longitudinal incision along the edge of the rectus muscle, with reflection of the peritoneum medially (Figure 9-3). In renal transplant patients, end-to-side renal artery anastomosis to the external iliac artery avoids division of the internal iliac artery. The efficacy of this procedure to prevent ED has been judged to be more effective by some groups than by others.48–50
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree