Chapter 70
Vascular Tumors of Childhood
Cameron C. Trenor III,, Arin K. Greene
Based on a chapter in the seventh edition by Glenn R. Jacobowitz
Vascular tumors of childhood are usually benign and consist of four major types: infantile hemangioma, congenital hemangioma, kaposiform hemangioendothelioma, and pyogenic granuloma.1,2 They are manifested during infancy or childhood, may involve any location, and can cause local complications: bleeding, destruction of tissue, obstruction, and pain. Systemic sequelae may include thrombocytopenia, congestive heart failure, and death.
Vascular tumors must be differentiated from vascular malformations of childhood. Vascular tumors usually are not present at birth, have proliferating endothelium, and exhibit postnatal growth. In contrast, vascular malformations arise from dysmorphogenesis, are present at birth, have quiescent endothelium, and grow proportionately with the child or slowly expand. Together, vascular tumors and malformations of childhood compose the field of vascular anomalies. The modern classification of vascular anomalies is based on cellular traits, correlated with physical findings and natural history3,4 (see Chapter 69).
The subject of vascular tumors of childhood is confusing because many practitioners still refer to vascular lesions by their descriptive rather than by their biologic term (Table 70-1).5 Consequently, communication, diagnosis, and treatment of patients with these lesions remain difficult. The suffix –oma describes a lesion that formed by upregulated cellular growth.3 Thus, this suffix is used for vascular tumors. Terms such as cystic hygroma (macrocystic lymphatic malformation), lymphangioma (microcystic lymphatic malformation), and cavernous hemangioma (venous malformation), which describe nonproliferating malformations, are discouraged.3
This topic is also challenging because many vascular tumors and malformations look alike. Lesions may appear flat or raised and blue, red, or purple. Management often requires interdisciplinary cooperation between medical and surgical specialties. Significant progress in understanding and treatment of patients with vascular anomalies has been made during the past quarter century. For example, imaging instead of biopsy is now the standard for diagnostic confirmation, antiangiogenic drug treatment is available for problem tumors, sclerotherapy has replaced operative resection for most malformations, and techniques for excision have been improved. Several multidisciplinary Vascular Anomalies Centers now serve as regional, national, or international referral centers for patients with these lesions.
Infantile Hemangioma
Clinical Features
Infantile hemangioma, a benign tumor of the endothelium, is the most common neoplasm of infancy. It affects about 4% to 5% of white infants and is rare in dark-skinned individuals.6 It is more frequent in premature infants (the risk is increased 40% for every 500-g decrease in birth weight less than 2500 g) and girls (4 : 1).7 Most are single (80%) and involve the head and neck (60%), trunk (25%), or extremity (15%). Between 30% and 50% of lesions are visible at birth as a small pale spot, telangiectatic stain, or ecchymotic area. However, the median age at appearance is 2 weeks after birth.
Infantile hemangioma has a unique growth pattern. During the first 9 months of life, the lesion grows rapidly, faster than the growth of the child (proliferating phase) (Fig. 70-1). Eighty percent of tumor growth is achieved by 3.2 (±1.7) months of age.8 If the tumor involves the superficial dermis, it appears red. If it occupies the deep dermis, the overlying skin may be bluish or have a normal appearance. By 10 to 12 months of age, growth plateaus and the tumor increases in size at the same rate of growth as the child. The involuting phase begins at approximately 1 year of age and is characterized by shrinkage of the tumor. The bright color fades, the skin pales at the center of the lesion, and the tumor becomes less tense. Involution is completed in most children by 3.5 years of age.9 Size, site, and previous treatment do not affect the rate of regression.9 The final involuted phase occurs after regression is complete. Fifty percent of patients have residually damaged skin, fibrofatty tissue, telangiectasias, discoloration, scarring, or redundant skin (Fig. 70-2).
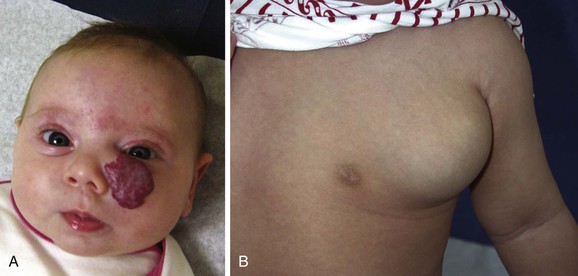
Figure 70-1 Proliferating infantile hemangioma. A, A 2-month-old girl with a superficial tumor. B, A 9-month-old girl with a deep lesion of the chest.
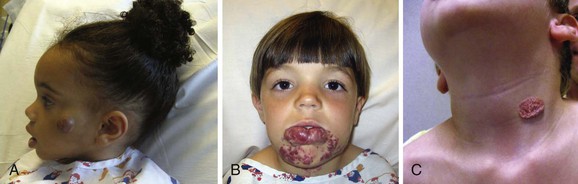
Figure 70-2 Involuting infantile hemangioma. A, A 3-year-old girl with a residual fibrofatty lesion of the cheek. B, A 4.5-year-old girl with fibrofatty residuum and telangiectasias of the lower lip. C, A 4-year-old boy with redundant skin of the neck.
Multiple Hemangiomas
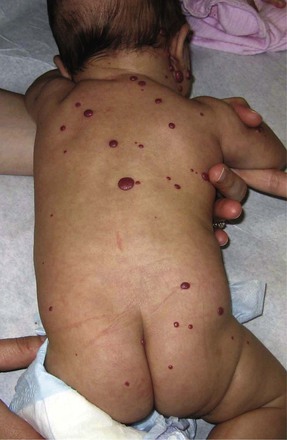
On occasion, children have multiple cutaneous hemangiomas (hemangiomatosis). The lesions usually are less than 5 mm in diameter and are domelike (Fig. 70-3). Individuals with five or more cutaneous hemangiomas have a 16% chance of having visceral lesions that are almost always located in the liver.10 Patients are screened with ultrasonography to rule out hepatic hemangiomas.11 The brain, gut, and lung are rarely involved.
Hepatic Hemangiomas
The liver is the most common site of extracutaneous hemangioma. Whereas most are small and discovered incidentally, children may present during the proliferating phase with congestive heart failure, hepatomegaly, anemia, or hypothyroidism. Differential diagnosis includes arteriovenous malformation and malignant neoplasm. However, 90% of fast-flow lesions of the liver are hemangiomas; the remaining fast-flow lesions are arteriovenous malformations.11 Hepatoblastoma and metastatic neuroblastoma are less common and do not illustrate the shunting characteristics of hemangioma.
Hepatic hemangiomas may be focal, multifocal, or diffuse.11 Focal lesions typically are asymptomatic and discovered incidentally on prenatal or antenatal ultrasonography. They usually are not associated with cutaneous hemangiomas. A focal lesion is a rapidly involuting congenital hemangioma and not an infantile hemangioma.11 Focal lesions undergo rapid involution postnatally and do not stain positive for GLUT1, a marker for infantile hemangioma.12 Rarely, macrovascular shunts from the hepatic artery or portal vein to the hepatic veins can cause congestive heart failure, possibly necessitating embolization of the shunts.
Multifocal hepatic hemangiomas also are usually asymptomatic and discovered incidentally. Rarely, multifocal tumors can cause high-output cardiac failure by arteriovenous or portovenous shunting. Unlike focal lesions, multifocal hepatic hemangiomas are infantile hemangiomas and may be associated with multiple cutaneous lesions. These lesions stain for GLUT1 and begin involution after 12 months of age.11
A diffuse hemangioma can replace hepatic parenchyma and cause massive hepatomegaly, although high-output cardiac failure is rare. However, the inferior vena cava or thoracic cavity may be compressed, causing respiratory compromise or abdominal compartment syndrome. Virtually all infants will develop hypothyroidism because the hemangioma expresses a deiodinase that inactivates thyroid hormone.13 Patients with diffuse hepatic hemangioma must have thyroid-stimulating hormone monitoring. Massive intravenous thyroid hormone replacement may be necessary to prevent irreversible mental retardation until the hemangioma regresses. Liver transplantation may be indicated in a critical situation if the hemangioma fails to respond to pharmacotherapy.
Lumbosacral Location
An infant with a large midline infantile hemangioma involving the lumbosacral area has an approximately 1 in 3 chance of having an underlying spinal anomaly (e.g., tethered cord, lipoma, intraspinal hemangioma).14 Magnetic resonance imaging (MRI) is performed between 3 and 6 months of age to rule out an occult spinal dysraphism.
PHACES Association
PHACES association (posterior fossa brain malformations, hemangioma, arterial anomalies, coarctation of the aorta and cardiac defects, eye abnormalities, sternal clefting/supraumbilical raphe) refers to a plaque-like infantile hemangioma of the face with one or more of the following anomalies: brain, cerebrovascular, cardiac, eye, and sternal/supraumbilical.15 Ninety percent of affected children are female, and the most commonly associated anomaly is a cerebrovascular malformation (72%).15 Less than one third of patients have more than one extracutaneous feature of the association. PHACES is estimated to represent 2.3% of all patients with infantile hemangioma, and 8% of patients with PHACES have infant stroke.15 MRI is obtained to evaluate the cerebrovasculature. If an anomaly is present, neurologic consultation is obtained. Ophthalmologic, endocrine, and cardiac evaluations are necessary to determine if associated anomalies are present.
LUMBAR Association
Two thirds of infants with LUMBAR association (lower body infantile hemangioma, urogenital anomalies/ulceration, myelopathy, bone deformities, anorectal malformations/arterial anomalies, and renal anomalies) are girls.16 The infantile hemangioma is large, superficial, and located in a regional distribution. The tumor has minimal postnatal growth and often ulcerates. The hemangioma affects the sacral area, lumbar region, perineum and genitalia, or lower extremity.16 Associated anomalies include spinal, cutaneous, anorectal, renal, urogenital, arterial, and bone. Infants younger than 3 months with suspected LUMBAR association undergo screening ultrasound of the spine, abdomen, and pelvis to determine whether a large anomaly or spinal dysraphism is present. Infants older than 3 months undergo screening MRI.16
Pathogenesis
Evidence suggests that infantile hemangioma may arise from vasculogenesis (formation of blood vessels from progenitor cells).17 The precursor cell for infantile hemangioma may be a multipotent hemangioma-derived stem cell, which has been isolated.18 These cells produce human GLUT1-positive vessels in immunodeficient mice.18 Hemangioma-derived stem cells share similarities with placental endothelium, although genetic studies have shown that hemangioma-derived stem cells are derived from the child and not the mother.19,20
Several mechanisms might contribute to the enlargement of infantile hemangioma. Hypoxia may stimulate circulating hemangioma-derived endothelial progenitor cell recruitment to the tumor. Hemangioma-derived stem cells have defective activity of nuclear factor of activated T cells and decreased expression of vascular endothelial growth factor receptor (VEGFR)–1.21 Because VEGFR-1 is a decoy receptor, more vascular endothelial growth factor A becomes available to bind to VEGFR-2, which stimulates endothelial proliferation.21 A reduction in local antiangiogenic proteins also may potentiate tumor growth.22,23
The mechanism for infantile hemangioma involution is unknown. As endothelial proliferation slows, apoptosis increases, and the tumor is replaced by fibrofatty tissue. Apoptosis begins before 1 year of age and peaks at 24 months.24 Decreasing proangiogenic maternal estrogens or increasing angiogenesis inhibitors in the epidermis overlying the hemangioma may promote involution.23 The source of adipocytes during involution is the hemangioma-derived stem cells, which also can differentiate into pericytes.18
Diagnosis
History and Physical Examination
Correct diagnosis of a vascular tumor of childhood can be made by history and physical examination in 90% of patients. When the diagnosis is unclear, radiographic imaging is usually diagnostic; biopsy is rarely necessary. Infantile hemangioma is not present at birth, although a light stain may be noted in 50% of infants. At an average age of 2 weeks, the lesion will grow rapidly larger. By 12 months of age, infantile hemangioma will begin to involute, becoming gray, soft, and smaller. A superficial infantile hemangioma appears red, whereas deeper lesions may not be noted until later as a blue mass visualized through the skin. A deep infantile hemangioma without significant cutaneous changes may be diagnosed with a handheld Doppler device; fast flow is consistent with infantile hemangioma.
Imaging
Less than 10% of infantile hemangiomas require imaging for a definitive diagnosis to be obtained. Ultrasound is the first-line confirmatory study and shows a well-circumscribed hypervascular mass.25 Low-resistance arterial waveforms are present with increased venous drainage. If ultrasound is equivocal, an MRI study is obtained. During the proliferative phase, an infantile hemangioma shows a parenchymal mass (unlike an arteriovenous malformation) with dilated vessels and signal voids. The lesion is isointense on T1 sequences, is hyperintense on T2 images, and enhances homogeneously after the administration of contrast material.25 An involuting infantile hemangioma has increased lobularity and adipose tissue. A reduced number of vessels, signal voids, and enhancement are noted.
Histopathology
Less than 1% of infantile hemangiomas require histopathologic evaluation for diagnosis. Biopsy is indicated if malignant disease is suspected or if the diagnosis remains unclear after the lesion is imaged. Rare lesions that may be confused with common vascular tumors of infancy include arteriovenous malformation, infantile myofibromatosis, infantile fibrosarcoma, Enzinger intramuscular hemangioma, pilomatrixoma, neuroblastoma, and lymphoma.26,27 A proliferating lesion shows tightly packed capillaries with plump endothelial cells and minimal intervascular stroma.28 During involution, infantile hemangioma exhibits reduced capillaries, enlargement of channels, and increased stroma. An involuted tumor is primarily fibrofatty with few residual capillaries.28 Infantile hemangioma uniquely expresses an erythrocyte-type glucose transporter (GLUT1) that can be used to differentiate the tumor immunohistochemically from other vascular tumors and malformations.12
Management
Observation
Observation is the mainstay of management because 90% of infantile hemangiomas are small, are localized, and do not involve aesthetically or functionally important areas.1 Parents should be reassured by showing them photographs of the tumor in its proliferative, involuting, and involuted phases. Patients are observed closely through the proliferative phase of growth if the tumor is at risk for becoming a problem (e.g., obstruction or destruction of important areas).
Wound Care
During the proliferative phase, approximately 16% of lesions will have skin ulceration.29 Ulceration is more common on the lips, neck, and anogenital regions. To reduce the risk of ulceration, tumors can be covered with hydrated petroleum. Lesions in high-risk areas may be further protected with a petroleum gauze barrier.1 Ulcerated infantile hemangiomas are treated with soap and water irrigations twice daily. Small areas are covered with topical antibiotic. Deep wounds are managed with damp-to-dry dressing changes.
Topical Pharmacotherapy
Topical pharmacotherapy has minimal efficacy, especially when the infantile hemangioma is primarily in the deep dermis and subcutis.1 Although the lesion may lighten, if an underlying mass is present, it will not be affected. Topical ultrapotent corticosteroid (e.g., clobetasol) may be effective for small, superficial lesions, but hypopigmentation and skin atrophy can occur. The beta blocker timolol may have efficacy for small, superficial lesions. It is typically applied twice daily, but systemic absorption of the drug can occur.30
Intralesional Corticosteroid
Intralesional administration of corticosteroids is indicated for small, well-localized infantile hemangiomas that obstruct vision or the nasal airway or are at risk for damaging an aesthetically important area (eyelid, lip, nose).1 Triamcinolone stabilizes growth in 95% of infantile hemangiomas, and 75% will decrease in size.31 Injections are administered at 6-week intervals during the proliferative phase. Risks include subcutaneous fat atrophy and ulceration. Blindness has been reported with injection of periorbital hemangioma that may have been due to embolic occlusion of the retinal artery. If a localized infantile hemangioma fails to respond to corticosteroid injection, systemic pharmacotherapy is considered (Fig. 70-4).
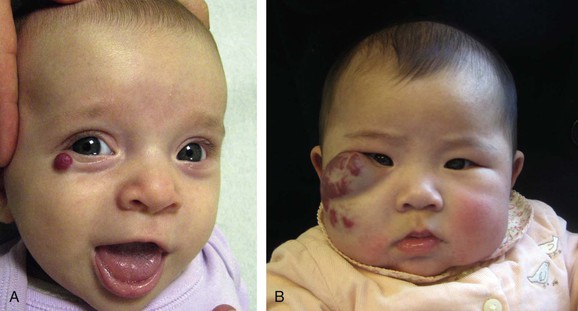
Figure 70-4 Corticosteroid treatment for problem infantile hemangiomas. A, A 2.5-month-old girl with a small periorbital lesion. To prevent amblyopia, the tumor was successfully treated with corticosteroid injection. B, A 4.5-month-old girl with obstruction of the visual axis. Because of its large size, treatment required oral corticosteroid administration.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree