Chapter 156
Vascular Trauma
Head and Neck
Benjamin W. Starnes, Zachary M. Arthurs
Based on a chapter in the seventh edition by Zachary M. Arthurs and Benjamin W. Starnes
Cervical vascular injuries are notoriously difficult to evaluate and to manage, mostly secondary to complex anatomy confined to a relatively narrow anatomic space. The initial evaluation of these patients is often obscured by associated injuries to the head, chest, or abdomen. In addition, signs of cerebral ischemia, cranial nerve deficits, or cervical nerve compression may not be present on initial evaluation. The evaluation and appropriate management of this injury pattern have been controversial and continue to evolve. Advances in noninvasive imaging (primarily computed tomography) have revolutionized the evaluation of stable patients with cervical vascular injuries, aerodigestive injuries, and associated fractures. In addition, endovascular surgery has added another facet to the care of these trauma patients. Injuries to the distal internal carotid artery, proximal common carotid artery, subclavian artery, or vertebral arteries are now amenable to endovascular methods to arrest hemorrhage, to exclude dissections and pseudoaneurysms, or to assist with open repair. This chapter addresses the presentation, evaluation, and treatment of cervical vascular injuries.
Carotid Arteries
Penetrating Injury
After penetrating cervical trauma, cervical blood vessels are the most commonly injured structures in the neck and account for a 7% to 27% stroke rate and a 7% to 50% mortality.1 In this population, 80% of deaths are stroke related.
Clinical Presentation
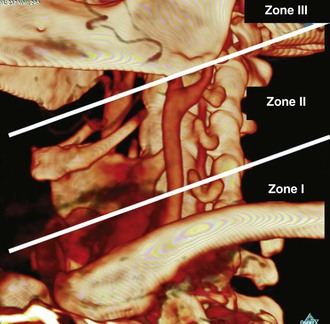
The neck has classically been divided into three zones that dictate diagnostic evaluation and treatment2 (Fig. 156-1):
• Zone I: below the cricoid cartilage—proximal control obtained in the chest.
• Zone III: above the angle of the mandible—distal control difficult to obtain.
Zone II is the most commonly injured (47%), followed by zone III (19%) and zone I (18%). It is not uncommon for the injury to traverse two zones of the neck.3 In addition to location, the physical examination triages patients on the basis of “hard signs” of vascular injury (mandating exploration) and “soft signs” of vascular injury (observation vs. further diagnostic evaluation). Hard signs include shock, refractory hypotension, pulsatile bleeding, bruit, enlarging hematoma, and loss of pulse with stable or evolving neurologic deficit. Soft signs include history of bleeding at the scene of injury, stable hematoma, nerve injury, proximity of injury track, and unequal upper extremity blood pressure measurements. Ninety-seven percent of patients with hard signs have a vascular injury as opposed to only 3% with soft signs.3
On the basis of mechanism of injury, gunshot wounds are more likely to cause a large neck hematoma and vascular injury (27%) compared with stab wounds (15%).3 Shotgun wounds, blast injuries, and transcervical (crossing midline) gunshot wounds have a higher rate of vascular injury and should be approached with a high index of suspicion. Associated injuries to the tracheobronchial tree, esophagus, and spinal cord are present in 1% to 7% of patients.3 In addition to hard signs of a vascular injury, patients may present with hard signs of a tracheobronchial injury (respiratory distress or air bubbling from the wound), mandating operative exploration. Other soft signs of cervical neck injury include painful swallowing, subcutaneous emphysema, hematemesis, and signs of nerve injury (cranial nerves IX, X, XI, and XII) or brachial plexus injury (axillary, musculocutaneous, radial, median, and ulnar nerves). A focused and detailed clinical evaluation reliably identifies patients with vascular injuries that require treatment. A physical examination with normal findings has a negative predictive value of 90% to 100% for vascular injuries.4
Special consideration should be given to patients who present with coma, a dense hemispheric stroke, or documented carotid thrombosis. The treatment of this specific injury pattern has come full circle from revascularization in the 1950s, to routine ligation in the 1970s, followed by revascularization as the current mainstay of treatment. In the 1970s, authors reported only a few patients with dense hemispheric stroke who developed hemorrhagic stroke after revascularization, leading to the recommendation of internal carotid artery ligation distal to the thrombus.5–7 Follow-up studies demonstrated that the extent of anoxic brain injury (not hemorrhagic conversion of the injury), development of reperfusion injury, cerebral edema, and resultant uncal herniation accounted for patients with worsening neurologic status and death.8,9 However, to date, there is no preoperative marker other than time (>24 hours from time of injury) that predicts those patients unlikely to benefit from revascularization. Early revascularization has consistently demonstrated improvement or stabilization of neurologic symptoms in patients with dense hemispheric strokes (100%), even in patients who present obtunded (50%).1,10
Diagnostic Evaluation
Patients with hard signs of a vascular injury should proceed to the operative suite. All patients should have plain radiographs of the neck and chest to determine the track of the injury and to diagnose occult hemothoraces or pneumothoraces. There have been several advances in the treatment of penetrating neck injuries, and data are now sufficient to support selective exploration in hemodynamically stable patients who do not have hard signs of a vascular or tracheobronchial injury. Exploration of cervical injuries based on platysma muscle penetration carries an unacceptably high negative exploration rate of 50% to 90%.11
Computed tomography is the modern workhorse for trauma evaluation and should be the initial diagnostic step in evaluating patients with penetrating neck injuries who do not have hard signs of vascular or aerodigestive injury. Contrasted axial imaging with reformatting software allows exact determination of the injury track, vascular injuries, proximity to the esophagus and trachea, spinal fractures and cord involvement, and extension into the head or chest (Fig. 156-2). In the setting of penetrating cervical injuries, computed tomographic angiography (CTA) has a 90% sensitivity and 100% specificity for vascular injuries that require treatment.12,13 CTA may be limited secondary to missile fragments (especially shotgun injuries) or bone fragments obscuring the cervical vasculature; arteriography should be used for these patients as a confirmatory study. Ultrasonography has been used for penetrating neck trauma, but its utility is limited to zone II neck injuries.14 In addition, subcutaneous air, fragments, and hematomas make ultrasound less reliable.
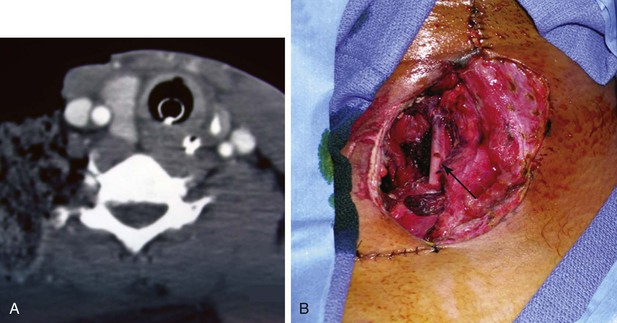
Figure 156-2 This patient sustained a high-velocity gunshot wound to zone I of the neck. On initial evaluation, he did not have hard signs of a vascular injury. A, CTA demonstrates no injury to the internal jugular vein or common carotid artery. In addition, there is no injury to the aerodigestive tract. B, The patient’s wound was débrided in the operative suite; the arrow marks the cords of the brachial plexus.
Medical Treatment (Nonoperative Management)
Occult injuries (intimal flaps, dissections, and pseudoaneurysms) identified during evaluation for penetrating cervical injury should be managed just as those caused by blunt trauma (detailed later). Isolated intimal flaps are rare in penetrating trauma, and dissections occur in only 2% of cases. Pseudoaneurysms are the most common occult injury identified. Large pseudoaneurysms should be considered for early intervention, whereas small pseudoaneurysms should be treated with antithrombotic therapy and early follow-up imaging. The natural history of these lesions is not known; however, patients should be closely monitored for development of embolic symptoms.
Endovascular Treatment
An endoluminal approach to neck injuries may avoid the morbidity of median sternotomy, a high thoracic incision, or difficult dissection at the base of the skull. Another benefit is that endoluminal therapy can be performed under local anesthesia, allowing the provider direct assessment of the patient’s neurologic status. For zone I and zone III injuries, endovascular exclusion of a pseudoaneurysm, partial transection, or arteriovenous fistula remains a viable option based on the location of injury and the patient’s clinical status. Self-expanding covered stents can be safely delivered to these locations with limited morbidity.15–18 Zone II injuries should be approached with operative repair.
Surgical Treatment
Proximal and Distal Control in the Neck.
Obtaining control of the injury in each zone presents unique challenges. All patients should have their proximal thighs (potential vein conduit) and chest (potential proximal control) prepared into the operative field. Zone I injuries that are manifested with hard signs may be approached through a cervical incision, but a median sternotomy or high anterolateral thoracotomy will be required to obtain proximal control. If the patient is in shock, endovascular attempts at proximal control should not delay performing a median sternotomy. Depending on the patient’s hemodynamics and the location of injury, proximal control of the great vessels may be performed from a femoral approach in the operative suite with balloon occlusion (a large 33-mm compliant balloon catheter). Alternatively, if the proximal vessel can be visualized from a cervical approach but not secured with a vascular clamp, a compliant balloon or Fogarty catheter can be passed retrograde for temporary proximal control. Once the vessel is properly exposed, the balloon can be replaced with a vascular clamp.
An overt injury in zone II can be readily approached through a cervical incision and repair performed under direct visualization. The most common vessel injured by penetrating mechanisms is the internal jugular vein followed by the common carotid artery. The operative feasibility, ability to examine the aerodigestive tract, and relatively low risk to exploration in this region favor open exploration over endovascular techniques in emergent situations.
Hemorrhage from a zone III injury can be devastating, and an immediate operative exploration through a cervical incision can be used first to control inflow and to assess the injury pattern. Even after subluxation of the mandible and division of the posterior belly of the digastric muscle, the distal extent of the injury may not be visualized. If the vessel is transected with inadequate length for clamp application, distal control can be obtained by placing a Fogarty balloon (No. 3-4) within the vessel lumen. If the vessel is lacerated, a sheath can be placed in the common carotid artery and a Fogarty catheter can be passed antegrade through the injury to control backbleeding. Once the Fogarty balloon is inflated, arteriography can be performed through the side arm of the sheath to delineate the injury with respect to the skull base and further guide operative exposure. Once hemorrhage is arrested, the surgeon must decide whether to proceed with operative repair, embolization of the carotid artery, endoluminal stenting, or temporary shunting or to return the patient to the intensive care unit for resuscitation, imaging of the brain, and delayed repair. If a damage control approach is used, the patient should have serial imaging to evaluate evolving cerebral edema, and cerebral perfusion pressures should guide further resuscitative maneuvers.
Surgical Repair of Cervical Vessels.
Once the injury has been delineated and controlled, the surgeon must decide whether to ligate, repair, or temporarily shunt the vessel. The internal jugular vein and external carotid artery may be ligated with limited morbidity. Ligation of the internal carotid artery results in a 45% mortality,1 and it should be reserved only for injuries at the base of the skull that are not amenable to reconstruction. Clean-based lacerations caused by stab wounds may be repaired primarily; however, gunshot wounds, fragmentation wounds, and shotgun injuries typically require reconstruction of the common carotid or internal carotid artery. Shunts should be used in patients who are already at risk of cerebral hypoperfusion secondary to shock and to all injuries of the internal carotid artery. If the distal clamp can be placed below the carotid bulb, the internal carotid artery will receive adequate backbleeding through the external carotid artery. Heparin (50 units/kg) should be given before clamps are placed.
The greater saphenous vein has good size match with the internal carotid artery and when used as an interposition graft has demonstrated excellent patency and limited infectious risk. The external carotid artery can also be transposed to the internal carotid artery for injuries in the proximal internal carotid. In addition, superficial femoral artery can be used in the common or internal carotid artery but requires an additional reconstruction in the lower extremity with polytetrafluoroethylene (PTFE).19 PTFE typically has a better size match than the greater saphenous vein in the common carotid artery, and in this location, there is no difference in patency rates between the two conduits. In the setting of associated aerodigestive injuries, autogenous conduits should be used, the esophageal repair should be drained away from the vascular repair, and a muscle pedicle (cervical strap muscles, omohyoid muscle, digastric muscle, or sternal head of the sternocleidomastoid) should be placed between the two repairs.
Blunt Cerebrovascular Injuries
The overall incidence of blunt cerebrovascular injury (BCVI) has been universally reported as less than 1% of all trauma admissions for blunt trauma, but this relatively small population of patients has stroke rates ranging from 25% to 58% and mortality rates of 31% to 59%.20–22 The variability in incidence of BCVI is 0.19% to 0.67% for unscreened populations compared with 0.6% to 1.07% for screened populations.20
Clinical Presentation
The recognition and treatment of BCVI have dramatically evolved during the past 2 decades. As imaging technology has improved with respect to both image quality and acquisition times, its use has become a fundamental diagnostic tool in blunt trauma evaluation. Paralleling advances in noninvasive imaging, a heightened awareness of BCVI has emerged. Through aggressive screening, these injuries have increasingly been recognized before devastating neurologic ischemia results.
Mechanism of BCVI.
Three basic mechanisms of injury are encountered: (1) extreme hyperextension and rotation, (2) a direct blow to the vessel, and (3) vessel laceration by adjacent bone fractures.23 The most common mechanism causing blunt carotid injury is hyperextension of the carotid vessels over the lateral articular processes of C1-3 at the base of the skull, which is typically a result of high-speed automobile crashes. There are also scattered case reports of chiropractic manipulation24 and rapid head turning with exercise causing BCVI.25 A direct blow to the artery typically occurs in the setting of a misplaced seat belt across the neck during a motor vehicle crash or in the setting of hanging. This injury pattern typically occurs in the proximal internal carotid artery as opposed to the distal aspect. Basilar skull fractures involving the petrous or sphenoid portions of the carotid canal can injure the vessel at this location.
Common mechanisms of injury associated with BCVI include motor vehicle crash (41%-70%), direct cervical blow (10%-20%), automobile versus pedestrian (12%-18%), fall from height (5%-15%), and hanging events (5%).20,22 Most common associated injuries at the time of diagnosis include closed head injuries (50%-65%), facial fractures (60%), cervical spine fractures (50%), and thoracic injuries (40%-51%).20,22
Signs and Symptoms of BCVI.
Case reports, as early as 1967, described BCVI with recognized symptoms of cerebral ischemia, and all patients were symptomatic at the time of diagnosis.26 Carotid injuries typically are manifested with a contralateral sensory or motor deficit, decreased mental status, or neurologic deficits not explained by closed head injury. A carotid-cavernous fistula may be manifested with orbital pain, proptosis, hyperemia, cerebral swelling, or seizure. Depending on whether the vessel is occluded or whether the resultant injury is a nidus for embolic events, the symptoms may be variable. Patients typically have coexisting traumatic brain injuries that may mask signs and symptoms of BCVI.
Patients may present to the trauma center with obvious signs of BCVI; however, many patients are initially asymptomatic and subsequently develop symptoms after a latent period. Several authors have reported times from 1 hour to several weeks after injury before the development of symptoms.27–30 Evaluating an unscreened trauma population, Berne et al found a median time to diagnosis of 12.5 hours for survivors of BCVI and 19.5 hours for nonsurvivors, suggesting a sufficient window of opportunity for diagnosis and treatment.22 Neither admission Glasgow Coma Scale score nor baseline neurologic examination correlates with subsequent development of symptoms attributed to BCVI.
Screening for BCVI.
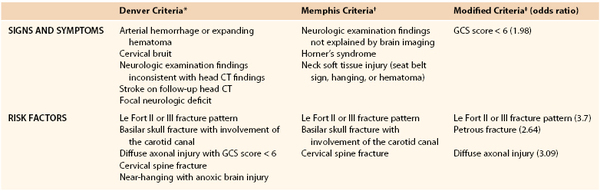
Although there is no consensus on the ideal screening protocol, several authors have found associations with signs, symptoms, and risk factors identified on admission. The first and most comprehensive screening protocol was initiated at the Denver Health Medical Center. The criteria are listed in Table 156-1.20,31 With this screening protocol, the authors reported an overall BCVI incidence of 0.86%. Exactly 4.8% of all trauma patients were screened on the basis of defined risk criteria, and 18% of screened patients were found to have an injury. Fifty-two percent of these screened patients were asymptomatic. Neurologic morbidity was 16%, and BCVI-associated mortality was 15%.20 Using the Memphis criteria (see Table 156-1), they found an incidence of 1.03%; 3.5% of all blunt trauma patients were screened, and 29% of screened patients were found to have an injury.32 Both screening regimens mandated four-vessel cerebral angiography if the patient met at least one of the screening criteria.
Several authors have evaluated a more restricted screening protocol in an effort to reduce the cost of screening and to limit the number of examinations with normal findings. A cervical seat belt sign has been evaluated in several prospective studies and has not been found to be predictive of BCVI.20,33 Biffl et al performed a multivariate analysis on a prospectively screened population and found four independent risk factors for BCVI listed in Table 156-1.34 Patients with one factor had a 41% risk of BCVI; two factors, 56% to 74%; three factors, 80% to 88%; and all four factors, 93%. However, 20% of patients with BCVI did not have any of the four risk factors. The bulk of the available literature supports an appropriate screening protocol for BCVI, and all major trauma centers should have predetermined screening criteria for BCVI.
Diagnostic Evaluation
Duplex Ultrasound.
Duplex scanning has been evaluated in multiple trauma centers for diagnosis of BCVI. In the evaluation of carotid artery stenosis, duplex ultrasound is limited when lesions of less than 60% stenosis are evaluated; likewise, duplex ultrasound will not often identify small intimal tears or nonocclusive dissections. It is often difficult to obtain adequate visualization of the internal carotid artery at the base of the skull, where the majority of these injuries occur. The sensitivity of duplex ultrasound for detection of BCVI ranges from 38% to 86%29,35; therefore, it should not be used as a screening modality.
Digital Subtraction Angiography.
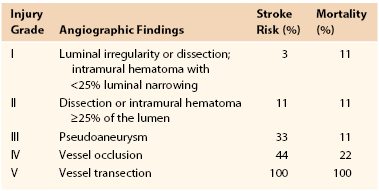
Selective digital subtraction angiography (DSA) is the diagnostic “gold standard” for screening patients with suspected BCVI. The Denver group proposed an angiographic grading system for BCVI (Table 156-2), which has become the standard for reporting BCVI.36 Most important, the grading scale held prognostic value for patients’ future risk of subsequent stroke.
There are several limitations of DSA that make it a difficult diagnostic tool. First and foremost, it is an invasive procedure with technical limitations and a complication profile that carries a risk of stroke (<1.0%).20 Performing screening DSA on all patients at risk of BCVI may impose a large economic and workload burden on the angiography suite; some institutions cannot support this type of demand.
Computed Tomographic Angiography.
Helical CTA offers several potential advantages over conventional DSA. It is a noninvasive study that can be obtained in less than 5 minutes, and as opposed to cerebral angiography, CTA obtains three-dimensional images of the vessel wall (Fig. 156-3). In addition, the workup of blunt trauma patients will inevitably involve CT imaging of the head, chest, abdomen, or all of these. CTA of the carotid-vertebral circulation can easily be obtained during this examination, sacrificing little in time (60 seconds per scan), contrast burden (approximately 100 mL), or radiation exposure.
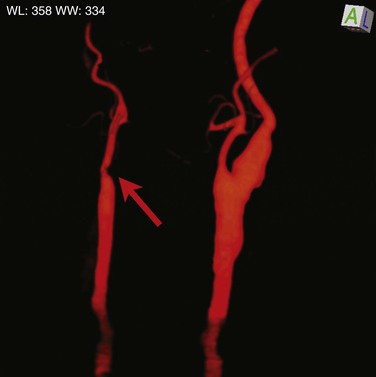
Figure 156-3 This three-dimensional CTA reconstruction with bone subtraction demonstrates occlusion of the right internal carotid artery. This patient presented with a zone II neck hematoma and a seat belt mark across the right side of the neck after an automobile crash. The arrow marks the occlusion at the origin of the internal carotid artery.
The ability to use CTA for screening depends on the quality of the scanner at the host institution. Using early-generation single-slice and four-slice CT scanners, two prospective comparative studies (performed by the Denver and Memphis groups) found CTA to have a sensitivity of 47% to 68% and specificity of 67% to 99% compared with DSA.32,37 CTA missed 55% of grade I, 14% of grade II, and 13% of grade III injuries.37 CTA technology rapidly improved during the ensuing years such that the number of detectors progressively increased and postimaging processing became readily available. Bub et al prospectively compared multidetector CTA (four- and eight-slice scanners) with DSA and found a sensitivity of 83% to 92% and a specificity of 88% to 98% from three different radiologists.38 The interobserver reliability was also higher for CTA than for DSA.
In 2005, Biffl et al reported their experience using 16-slice CTA for the diagnosis of BCVI, and contrary to the prior study in Denver disqualifying early-generation CT scanners, DSA was not used as the gold standard.39 During an 11-month period, 331 patients were screened, and 5.4% were diagnosed with BCVI. In this final study, CTA scans with normal findings were followed with clinical observation, and no patients developed neurologic symptoms consistent with delayed presentation of missed BCVI. All abnormal examination findings were confirmed with DSA. Of the 18 injuries identified by CTA, 17 were correctly graded, whereas one patient was upstaged to grade III on DSA (a small pseudoaneurysm was not identified on CTA; false-positive rate of 1.2%).39 In 2006, a prospective comparative study validated 16-slice CTA as a primary screening modality for BCVI.40 In this report, CTA was performed in addition to DSA in 162 consecutive patients; 20 carotid and 26 vertebral injuries were diagnosed. In that population, this resulted in an incidence of 1.25% and a screening yield of 28%, which is comparable to historic controls. CTA and DSA were 100% concordant for blunt carotid injuries, resulting in a sensitivity of 100% and a specificity of 100%.40 This is the only study comparing conventional 16-slice CTA directly with DSA and demonstrating equivalence. On the basis of these findings, a minimum of 16-slice CTA should be considered the primary screening modality for BCVI. Many authorities, however, still remain passionate that DSA should be the primary screening test of choice for patients with BCVI.41
In 2009, the initiation of a CTA-based screening and diagnostic program at one institution, along with interdisciplinary standardized treatment guidelines, reduced the time to diagnosis of BCVI 12-fold and the institutional stroke rate due to BCVI 4-fold. The authors concluded that this effect may be due to earlier diagnosis and initiation of definitive therapy.42
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree